Abstract
Background:
Specific-sized species of the carbohydrate hyaluronan elicit a variety of cellular responses mediating tissue integrity and repair, as well as regulating inflammatory responses. Orally provided hyaluronan with an average molecular weight of 35kDa (HA35) protects mice from short term ethanol-induced liver injury. This protection was associated with maintenance of the co-localization of zonula occludins-1 (ZO-1) and occludin at tight junctions in the proximal colon. However, it is not known whether HA35 also protects other regions of the intestine or whether protection is due to a direct and/or indirect interaction of HA35 with the intestinal epithelium.
Methods:
Female C57BL/6J mice were fed an ethanol containing diet or pair-fed control diet (4 days) and treated with or without HA35 via daily gavage during the last 3 days of ethanol feeding. Intestinal morphology and tight junction integrity was assessed. Differentiated Caco-2 cells were transfected or not with scrambled siRNA or siRNA targeting layilin, a hyaluronan receptor. Caco-2 cells were treated with or without HA35 prior to challenge with ethanol. Localization of tight junction proteins, FITC-dextran permeability and transepithelial electrical resistance (TEER) were evaluated.
Results:
While short-term ethanol did not result in any apparent changes in the gross morphology of the intestine, co-localization of ZO-1 and occludin at tight junctions was decreased in proximal and distal colon. HA35 prevented these effects of ethanol. In differentiated Caco-2 cells, ethanol decreased the localization of ZO-1 and occludin at tight junctions and increased permeability of FITC-dextran. At higher concentrations, ethanol also decreased TEER. Pre-treatment with HA35 prevented these changes. When the hyaluronan receptor Layilin was knocked down in Caco-2 cells, HA35 no longer protected cells from ethanol-induced loss of tight junctions.
Conclusion:
Taken together, these data indicate that HA35 interacts with Layilin on intestinal epithelial cells and maintains intestinal tight junction integrity during short-term ethanol exposure.
Keywords: alcoholic liver disease, intestinal epithelial cells, hyaluronic acid, tight junctions, Caco-2
Introduction:
Maintenance of the barrier function of the intestinal epithelium is critical for optimal gastrointestinal function, simultaneously allowing for the uptake of nutrients while preventing the translocation of pathogens and toxins (Shen, 2012). The paracellular interactions of tight junction proteins are essential for maintaining optimal function of the epithelial barrier (Forster, 2008). Ethanol consumption disrupts gastrointestinal tight junctions, allowing the leakage of bacterial lipopolysaccharide, peptidoglycan and other pathogen associated molecular patterns (PAMPs) into the circulation and to the liver (Chen et al., 2016). Increases in the portal concentration of LPS and other PAMPs stimulate resident macrophages in the liver to produce inflammatory mediators (such as tumor necrosis factor α (TNFα), monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6)) important for the development of liver injury and progression of alcoholic liver disease (ALD) (Gao et al., 2019).
Hospitalization and death from alcohol abuse are an increasing public health concern. Alcohol consumption has risen over the past 60 years and is projected to continue growing based on previous and current rates of consumption (Guirguis et al., 2015). Within the last 5 years alcohol has been recognized as the primary indication for cirrhotic deaths in the United States (Guirguis et al., 2015). Given the dramatic improvements in the treatment of hepatitis C combined with increasingly high rates of alcohol consumption, alcohol-related liver disorders are a leading cause of liver disease (Guirguis et al., 2015). However, current therapies to treat ALD, such as glucocorticoids or pentoxifylline, that target inflammatory responses are ineffective in most patients and limited to patients with end stage disease (Rambaldi et al., 2008). Given the critical interaction between the impact of ethanol on gut integrity and the progression of ethanol-induced liver injury, it is likely that therapeutic strategies targeted at protecting the barrier function of the intestinal epithelium would be efficacious at preventing the progression of ALD. Indeed, current clinical trials ( and ) in moderate and severe alcoholic hepatitis incorporate strategies aimed at improving gut health, including supplementation with zinc (McClain et al., 2017, Mohammad et al., 2012) and supernatants of Lactobacillus GG (Wang et al., 2012).
Hyaluronan is a ubiquitous constituent of the extracellular matrix involved in the maintenance of tissue integrity (de la Motte, 2011). Hyaluronan is synthesized at the plasma membrane as a linear unmodified polymer composed of the repeating disaccharides N-acetyl glucosamine and glucuronic acid and ranges in size from a 400 dalton disaccharide to polymers >1 million daltons in size. In addition to the well-defined role of high molecular weight hyaluronan as a structural molecule, smaller, defined molecular weight hyaluronan molecules can influence cellular responses through size-dependent interactions with hyaluronan receptors, including Layilin, CD44, receptor for hyaluronan mediated motility (RHAMM), toll-like receptor (TLR)2 and TLR4 (Cyphert et al., 2015). A number of lines of evidence indicate that hyaluronan is important for the maintenance of intestinal health. For example, hyaluronan is a component of breast milk and contributes to the establishment of intestinal health in newborns (de la Motte and Kessler, 2015, Hill et al., 2013). Further, specific-sized hyaluronan of an average molecular weight of 35kD (HA35) protects mice from Salmonella- (Kessler, 2018) and Citrobacter- (Kim et al., 2017) mediated disruption of the intestinal barrier and induces the expression of zonula occludens-1 (ZO-1).
Recent data from our laboratory demonstrates that HA35 also protects mice from short term ethanol-induced liver and gut injury; protection was associated with maintenance of tight junctions in the proximal colon during short-term ethanol feeding (Saikia et al., 2017). However, it is not known whether HA35 protects multiple regions of the intestine and whether its therapeutic efficacy is due to direct and/or indirect interactions with the intestinal epithelium. Therefore, the purpose of this study was to determine if HA35 can act directly on intestinal epithelial cells to maintain ZO-1 and occludin during ethanol challenge, as well as to identify the specific HA receptor responsible for the protective effects of HA35.
We report that short-term ethanol feeding promoted focal disruption of ZO-1 and occludin co-localization in both the proximal and distal colon; loss of co-localization was prevented in mice provided with HA35. Exposure of differentiated Caco-2 cells to ethanol in culture modeled the in vivo ethanol-induced disruption of tight junctions; treatment of Caco-2 cells with HA35 maintained the tight junction during ethanol challenge. siRNA-mediated knockdown of the hyaluronan receptor Layilin prevented HA35-dependent maintenance of tight junctions during challenge with ethanol. Taken together, these data suggest that HA35 might be a valuable therapeutic option to maintain intestinal epithelial barrier function in patients with ALD.
Methods:
Materials
Female C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). The Lieber-DeCarli high-fat liquid diet (#710260) was from Dyets, Bethlehem, PA. Caco-2 cells were purchased from the American Type Culture Collection (Manassas, VA). Hyaluronan with a molecular weight distribution of 21–40kDa (HA35)(catalog # HA20K-5) was from Life Core Biomedical (Chaska, MN). Primary antibodies were purchased from the following sources: rabbit anti-ZO-1 (Life Technologies, 61–7300), guinea pig anti-occludin (Hycult HP9047), rabbit anti-ß-actin (Cell Signaling, 4967), rabbit anti-Layilin (Bioss, 1842R). Secondary antibodies included donkey anti-rabbit 488 (A21206) and goat anti-guinea pig 568 (A11075) from Molecular Probes, Thermofisher Scientific (Waltham, MA) and goat anti-guinea pig horse radish peroxidase (sc-2438) from Santa Cruz (Dallas, TX). On-target plus smart pool siRNA directed against Layilin was obtained from Dharmacon (Lafayette, CO). Caco-2 cells were transfected using DharmaFECT 2 (Lafayette, CO). Caco-2 cells were cultured in 6.5mm transwell filters (Corning, 3.0 μm pore size) purchased from Thermofisher Scientific (Waltham, MA). Transwell filters for imaging studies were coated with 200 μl of a 0.004% solution of rat-tail collagen (Sigma-Aldrich, St Louis, MO) and transwells used for TEER were coated with type I collagen (Corning, #354236) dissolved in ethanol.
Murine model of short-term ethanol feeding
Ten to twelve week old C57BL6/J mice were housed two per micro-isolator cage and acclimated to the Lieber-DeCarli liquid diet for 2 days. Ethanol was included in the liquid diet at a concentration of 5% of calories (1% v/v) for 2 days and then increased to 32% of calories (6% v/v) for the last 2 days of the study. Maltose dextrin was isocalorically substituted in place of ethanol for pair-fed controls. During the last three days of the study, mice were treated with 15 mg/kg HA35 or an equivalent volume of saline by gavage at 1:30 pm (Saikia et al., 2017). The morning after the last HA35 treatment, ethanol- and pair-fed mice were anesthetized and samples collected prior to euthanasia. The intestine was excised and jejunum, ileum, proximal and distal colon were collected and fixed at room temperature in Histochoice (Amresco, Solon, OH) for 24 hours before paraffin blocking and sectioning. All animal procedures were approved and performed in accordance with the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Caco-2 monolayer cell culture and siRNA transfection
Caco-2 cells (passage 25–40) were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids. Caco-2 cells were plated in 6.5 mm Corning transwell filters (3 μm) at a density of 1 × 105 cells/transwell (for TEER and imaging), glass coverslips at 0.4 × 106 cells/slide (for imaging) or in 24 well plates at 1 × 105 cells/well (for Western blots) and differentiated for 21–23 days. Culture medium was changed every two to three days. For siRNA transfections, Caco-2 cells were differentiated for 20 or 21 days and then transfected with scrambled siRNA or siRNA directed against Layilin using Dharmafect 2 reagent (Lee et al., 2014). Small interfering RNAs were diluted in RNase free water to achieve a concentration of 5 μM and then diluted in OptiMEM to obtain a final siRNA concentration of 0.25 μM. The transfection reagent was prepared by mixing 50 μl of the siRNA in OptiMEM with 50 μl of prepared Dharmafect reagent (5% v/v diluted in OptiMEM). The Caco-2 medium was then replaced with 100 μl of the transfection reagent and incubated for 4 hours before overnight recovery in DMEM containing 10% v/v fetal bovine serum without penicillin-streptomycin. 24 h after transfection, the medium was replaced with DMEM containing all supplements.
Treatment with HA and ethanol and measurement of FITC-dextran permeability and TEER
Caco-2 monolayers were treated with 0.1 −1 mg/ml HA35 (as indicated in the Figure legends). After 24 hrs, ethanol was added to the medium to a final concentration of 40 mM, 500 mM or 1 M for an additional 3 hours (as indicated in the Figure legends). In some experiments, cells were pre-treated with 0.1–1 mM 4-methylpyrazole 1 h prior to challenge with ethanol. The permeability of the Caco-2 monolayers was assessed using FITC-labeled dextran (4 kDa). After treatment with HA35 and 40 mM ethanol, as described above, the upper and lower chambers of the transwells were washed three times with Dulbecco’s PBS and then 800 μl of Dulbecco’s PBS was added to the lower chamber and 150 μl of a 1 mg/ml FITC-dextran solution in Dulbecco’s PBS added to the upper chamber. The media from the upper and lower chambers was sampled after 1h and 2h and relative fluorescence measured in a multi-mode microplate reader at excitation 485 nm and emission 544 nm to assess macromolecular flux of FITC-dextran (SpectraMax, Molecular Devices, Sunnyvale, CA). For measurements of TEER, resistance was measured immediately before treatment with ethanol (t = 0) and following 3 h incubation using an EVOM2™ chop-stick electrode from World Precision Instruments.
Immunofluorescence of tight junctions
Immunofluorescent staining of tight junction proteins in the jejunum, ileum, proximal and distal colon (Kim et al., 2017) and Caco-2 monolayers (Rezaee et al., 2011) was performed. Paraffin-embedded intestinal sections were deparaffinized. Caco-2 cells were fixed in ice-cold methanol for 15 min and then washed three times with PBS for 5 min. Intestinal sections or Caco-2 monolayers were blocked in 2% BSA diluted in PBS for 1 hour at room temperature and then incubated overnight at 4°C in primary antibodies (ZO-1 (1:100) and occludin (1:50). Slides were then washed three times with PBS for 5 min and incubated with secondary antibody (1:250) for 2 hours at room temperature. Slides were mounted using Vectashield with DAPI (Vector laboratories, Burlingame, CA). Intestinal sections were imaged at 40X on an SP8 inverted confocal microscope. Caco-2 cells grown on glass coverslips were imaged at 20X on an Olympus BX61 microscope. Caco-2 cells cultured on transwell filters were imaged at 63X with a 3X digital magnification on a Leica TCS SP5 II confocal/multi-photon high-speed microscope. A 5.5μm Z-series encompassing the Caco-2 tight junctions was collected and compressed into a single 2D maximum image for each Caco-2 monolayer. Slides were coded and images acquired with a minimum of two images per slide. Slides incubated without the addition of primary antibody were used to correct for non-specific binding of the secondary antibodies. Immunoreactive ZO-1 or occludin staining in Caco-2 cells was semi-quantified by measuring the area of the fluorescent signal at the cellular interface divided by the skeletal length of the interface using ImageJ Software (Schindelin et al., 2012)
Immunoblotting analysis of tight junction proteins and Layilin
Protein lysates were prepared by first washing cells in PBS, followed by lysis for 10min on ice in a buffer containing 1% Triton X-100, 50mM Tris-HCl, 150 mM NaCl, 1mM EDTA, 0.1% sodium deoxycholic acid, 0.1% sodium dodecyl sulfate, 1 mM phenylmethanesulfonyl fluoride and Pierce Protease and Phosphatase Inhibitor (1 tablet/10ml of working lysis buffer) (Thermofisher, 88668). Protein lysates were sonicated for 10 seconds and then centrifuged at 13,000 × g for 10 min at 4°C before collecting the supernatants. Samples were diluted 1:5 or 1:10 to perform the DC Lowry Protein Assays and then diluted to 1.5 mg/ml in Laemlli buffer (Pritchard et al., 2007), flash frozen in liquid nitrogen and stored at −80°C. Immunoblotting was performed as previously reported (Mandal et al., 2010). PVDF membranes were incubated with primary antibody against occludin (1:500) and then probed with anti-guinea pig secondary antibody (1:5000). β-actin was used as a loading control.
Statistical Analysis
Differences between samples were assessed by ANOVA using the general linear model (SAS, Carey, IN). Multiple comparisons were analyzed using the least square means test. A significance threshold of P<0.05 was used to determine differences between comparisons.
Values represent means ± SEM.
Results:
Morphology of the intestine was not affected by short-term ethanol feeding or HA35
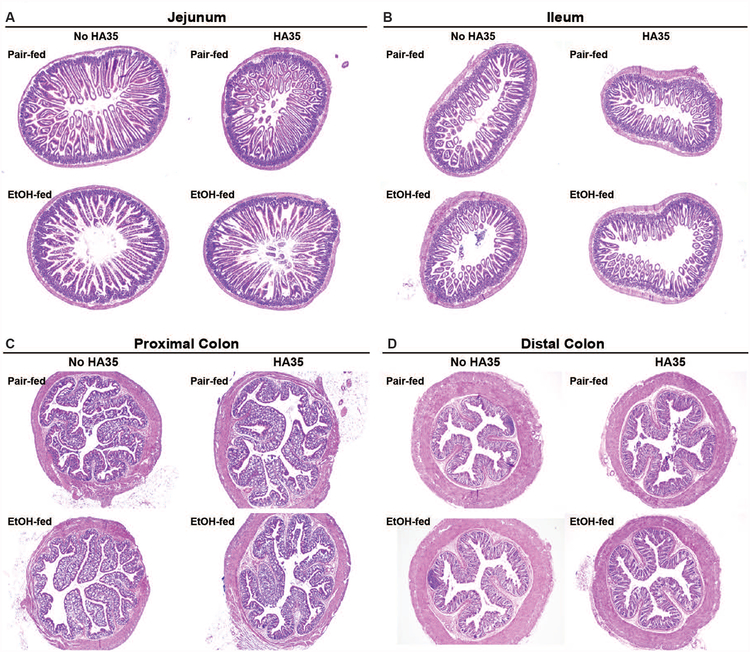
While chronic ethanol consumption results in changes the morphology of the intestine, characterized by erosion of the villus epithelium in the jejunum, decreases in villus height in the small intestine (Persson, 1991) and mononuclear cell infiltration in the colon (Shukla et al., 2016), in the short-term ethanol feeding model used in the current study, no overt morphological changes were observed in the intestine (Figure 1A–D).
Figure 1: Histology of the intestine in response to short-term ethanol feeding and treatment with HA35.
C57BL/6J mice were allowed free access to an ethanol containing diet (2 days at 11% of calories as ethanol then 2 days at 32% of calories as ethanol) or pair-fed an isocaloric control diet. Mice were provided with 15mg/kg body weight HA35 or an equivalent volume of saline by gavage during the last three days of the short-term ethanol feeding protocol. Paraffin-embedded sections of formalin-fixed (A) jejunum, (B) ileum, (C) proximal colon and (D) distal colon were de-paraffinized and stained with hematoxylin and eosin. Images acquired at 4X magnification. Images are representative of duplicate images captured from n=4 pair-fed or n=6 ethanol-fed mice in each treatment group.
HA35 maintained tight junctions during ethanol feeding
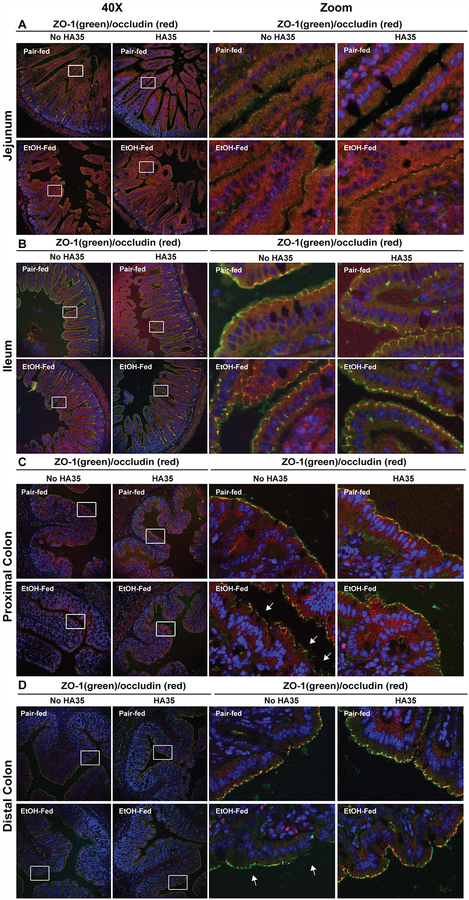
Intestinal permeability increases early in the progression of ALD and involves the disruption of epithelial tight junctions within the gastrointestinal tract (Elamin et al., 2014b). Protection of tight junctions in the proximal colon of mice by treatment with HA35 was associated with protection from ethanol-induced liver injury in mice (Saikia et al., 2017). Here we have extended this analysis to determine the interactions between ethanol and HA35 on the tight junction proteins ZO-1 and occludin in other intestinal regions. Short-term ethanol feeding had no effect on the co-localization of ZO-1 and occludin in the jejunum and ileum (Figure 2A and B). However, short-term ethanol feeding led to focal disruptions in the localization of ZO-1 and occludin at paracellular junctions in both the proximal and distal colon (Figure 2C and D). Treatment of mice with HA35 protected both the proximal and distal colon from the ethanol-induced disruption in the co-localization of ZO-1 and occludin (Figure 2C and D). Taken together, these data suggest that both the proximal and distal colon were sensitive to early ethanol-induced changes in localization of ZO-1 and occludin at tight junctions and that orally delivered HA35 was sufficient to prevent this disruption.
Figure 2: HA35 treatment prevented the short-term ethanol-induced disruption of ZO-1 and occludin co-localization at tight junctions in proximal and distal colon.
C57BL/6J mice were allowed free access to an ethanol containing diet (2 days at 11% of calories as ethanol then 2 days at 32% of calories as ethanol) or pair-fed an isocaloric control diet. Mice were provided with 15mg/kg body weight HA35 or an equivalent volume of saline by gavage during the last three days of the short-term ethanol feeding protocol. Immunostaining for ZO-1 (green) and occludin (red) in deparaffinized sections of (A) jejunum, (B) ileum, (C) proximal colon and (D) distal colon fixed with HistoChoice. Nuclei were visualized with DAPI (blue) staining. Images were acquired at 40X magnification and are representative of duplicate images captured from n=4 pair-fed or n=6 ethanol-fed mice in each treatment group. White boxes indicate representative areas of tight junctions for each intestinal region that were digitally magnified for improved visibility.
Culture of Caco-2 monolayers with HA35 prevented ethanol-induced disruption of tight junctions and normalized permeability and TEER
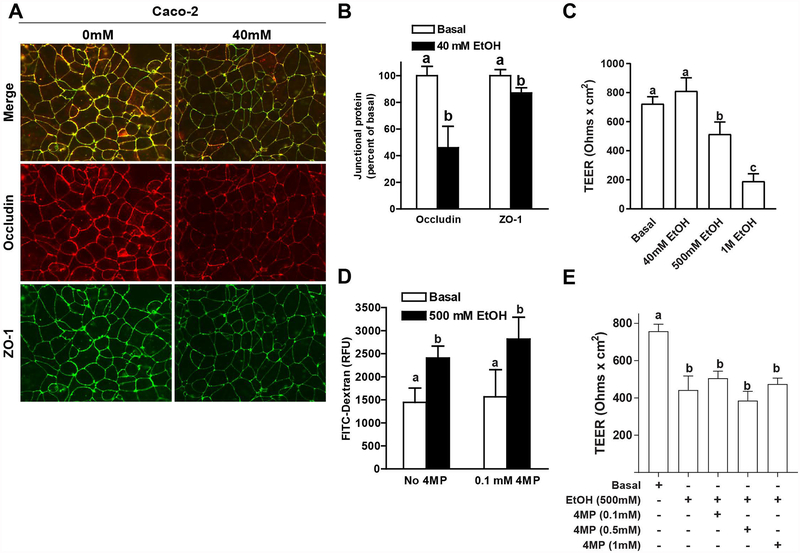
Since orally provided HA35 was sufficient to prevent ethanol-induced disruption of tight junctions in the colon of mice, Caco-2 cells were used to test the hypothesis that HA35 maintains tight junctions through direct interaction with epithelial cells. Caco-2 cells were differentiated and then cultured with or without 40 mM ethanol for 3 hours to induce disruption of tight junctions (Elamin et al., 2014a). Following challenge with 40 mM ethanol, the localization of ZO-1 and occludin at the junctional complex was decreased (Figure 3A). Immunoreactive occludin at the paracellular junctions was reduced by approximately 50%, while immunoreactive ZO-1 was only modestly decreased at the junctions by challenge with 40 mM ethanol. Measures of TEER were used to assess the impact of ethanol on the functional integrity of tight junctions in Caco-2 cells. Interestingly, 40 mM ethanol did not decrease TEER in differentiated Caco-2 cells (Figure 3C). However, 500 mM and 1 M ethanol dose-dependently decreased TEER (Figure 3C). Inhibition of ethanol metabolism with the addition of 4-methylpyrazole did not effect ethanol-induced decrease in FITC-dextran permeability (Figure 3D) or TEER (Figure 3E).
Figure 3: Exposure of differentiated Caco-2 monolayers to ethanol decreased the co-localization of ZO-1 and occludin at tight junctions and transepithelial electrical resistance (TEER).
(A/B) Caco-2 monolayers were differentiated for 21 days and then challenged with 40 mM ethanol for 3 hours. (A) Cells were fixed in methanol and immunoreactive ZO-1 (green) and occludin (red) visualized by fluorescence microscopy. Images were acquired at 20X magnification and are representative of at least two images per slide and three independent experiments. (B) Fluorescent intensity at the cellular interface was semi-quantified for occludin and ZO-1 using Image J software. (C) Differentiated Caco-2 monolayers were challenged with 40 mM, 500 mM or 1 M ethanol for 3 h. TEER was measured immediately before the addition of ethanol and again after 3 h. (D/E) Differentiated Caco-2 cells were pre-treated with or without 0.1–1 mM 4-methylpyrazole (4-MP) for 1 h prior to challenge with 500 mM ethanol. (D) FITC-dextran (4Kd) was added to the upper chamber of the transwells and leakage into the lower chamber assessed at 1 and 2h. (E) TEER was measured immediately before the addition of ethanol and again after 3 h. Values are expressed as means ± SEM, (A,B) n=3, (C) n=3–10, (D) n=3–4 and (E) n=4–8. Values with different alphabetical superscripts were significantly different from each other, p < 0.05.
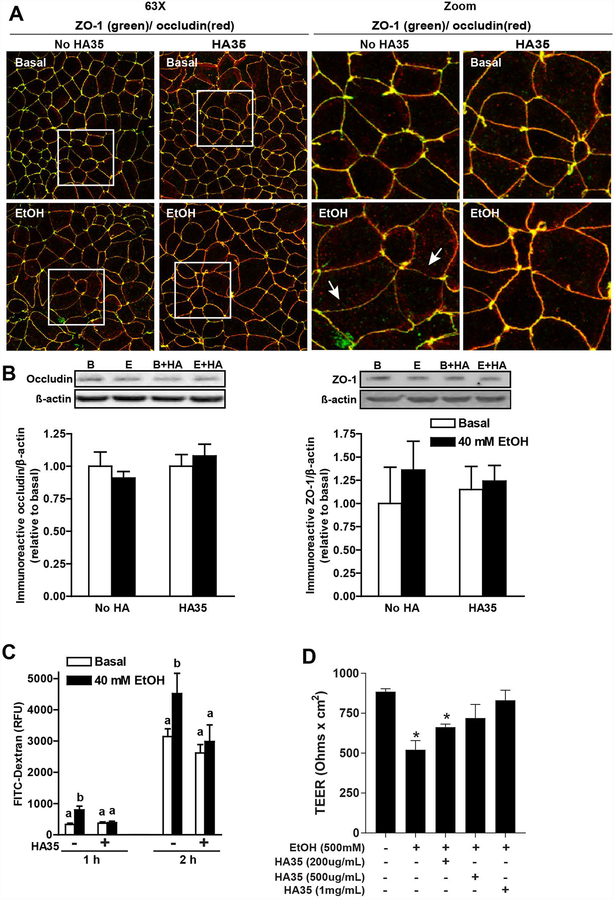
To determine whether culture of Caco-2 cells with HA35 would protect tight junctions from the effects of ethanol, differentiated Caco-2 monolayers were treated with or without 100 μg/ml HA35 for 24 h prior to challenge with ethanol. HA35 pre-treatment maintained the localization of ZO-1 and occludin at the paracellular junction in response to 40 mM ethanol challenge (Figure 4A), without affecting the total expression of occludin or ZO-1 protein, as assessed by immunoblotting (Figure 4B). Challenge of Caco-2 monolayers with 40 mM ethanol increased the permeability of FITC-dextran; pre-treatment with HA35 prevented the ethanol-induced increase in permeability (Figure 4C). HA35 was also able to prevent the decrease in TEER observed in response to 500 mM ethanol; this protection was dose-dependent, with full protection observed at 0.5–1 mg/ml HA35 (Figure 4D). Taken together, these data indicate that HA35 can directly interact with Caco-2 cells to maintain the localization of ZO-1 and occludin at tight junctions and maintain normal TEER during ethanol exposure.
Figure 4: Culture of differentiated Caco-2 monolayers with HA35 prevented ethanol-induced disruption of tight junctions.
(A/B/C) Caco-2 monolayers were differentiated for 21 days and then treated or not with 100 μg/ml HA35 for 24 hours, followed by challenge with or without 40 mM ethanol for 3 hours. (A) Cells were then fixed in methanol and immunoreactive ZO-1 (green) and occludin (red) visualized by confocal microscopy. Images acquired at 63X with a 3X digital magnification and are representative of at least two images per slide from three independent experiments. (B) Occludin and ZO-1 expression was assessed by immunoblot using ß-actin as a loading control. Semi-quantification of protein expression was performed using Carestream Imaging Software. (C) FITC-dextran (4Kd) was added to the upper chamber of the transwells and leakage into the lower chamber assessed at 1 and 2h. (D) Differentiated Caco-2 monolayers were treated with increasing concentrations of HA35 for 24 h and then challenged with 500 mM ethanol. TEER was measured immediately before the addition of ethanol and 3 h later. Values are expressed as means ±SEM, (A) n=3, (B) n=4–6 and (C) n=12 (values with different superscripts are significantly different within a time point, p<0.05) and (D) n=4 (*p < 0.05 compared to cells not treated with HA35)
HA35 maintained epithelial tight junctions during ethanol challenge through a Layilin-dependent mechanism
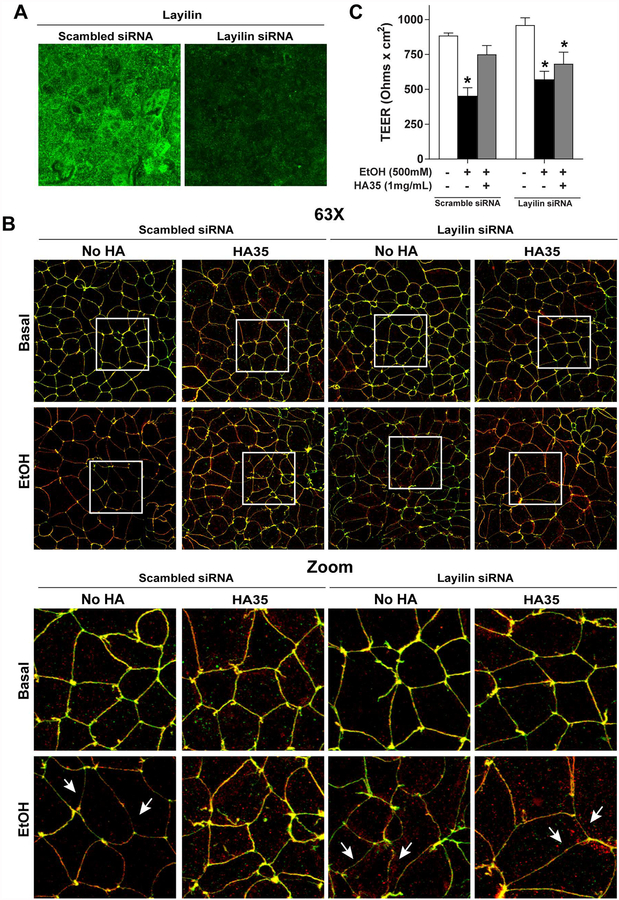
Epithelial cells express multiple hyaluronan receptors, including the highly expressed hyaluronan receptor Layilin. Layilin, which recognizes specific small sized HA fragments (Forteza, et al., 2012), is implicated in maintaining membrane-cytoskeletal junctions important for tight junctions (Arpin et al., 2011, Bono et al., 2005), forming complexes with cytoskeletal-membrane linker proteins, including talin, merlin and radixin, proteins important for the organization of cell-cell junctions (Bono et al., 2005, Borowsky and Hynes, 1998). The role of Layilin in mediating the effects of HA35 in the maintenance of tight junctions during ethanol exposure was assessed in Caco-2 cells using a siRNA-mediated knockdown strategy. Layilin was highly expressed at the apical surface of differentiated Caco-2 cells, as well as in a punctate pattern within the cytosol (Figure 5A). Transfection with Layilin-siRNA resulted in ~65% knockdown of Layilin expression in differentiated Caco-2 monolayers as compared cells transfected with scrambled siRNA (Figure 5A). Transfection of the Caco-2 cells did not affect baseline co-localization of ZO-1 and occludin (Figure 5B) or TEER (Figure 5C). Similarly, the effect of ethanol on ZO-1 and occludin localized at the junctions (Figure 5B) and TEER (Figure 5C) was equivalent in cells transfected with scrambled siRNA and siRNA targeting Layilin. As in non-transfected Caco-2 cells (Figure 4), in cells transfected with scrambled siRNA, 100 μg/ml HA35 protected co-localization of ZO-1 and occludin at the junction in cells challenged with 40 mM ethanol (Figure 5B). Pre-treatment with 1 mg/ml HA35 also maintained TEER after challenge with 500 mM ethanol in cells transfected with scrambled siRNA (Figure 5C). In contrast, HA35 did not protect cells transfected with siRNA targeting Layilin (Figure 5B/C).
Figure 5: siRNA-mediated knockdown of Layilin eliminated the ability of HA35 to protect tight junctions during challenge with ethanol in Caco-2 cells.
Caco-2 monolayers were differentiated on collagen-coated transwell filters and then transfected with scrambled siRNA or siRNA targeting Layilin. (A) Immunofluorescent staining for Layilin 24 h following transfection with scrambled siRNA or siRNA targeting Layilin. Images are representative of at least two images per slide from three independent transfections. (B) 24 h after transfection, cells were treated or not with 100μg/ml HA35 for 24 hours and then challenged with or without 40mM ethanol for 3 hours. Immunoreactive ZO-1 (green) and occludin (red) was visualized by confocal microscopy in cells transfected with scrambled siRNA or siRNA targeting Layilin. Images acquired at 63X with a 3X digital magnification and are representative of at least two images per slide from four independent experiments. (C) Cells transfected with scrambled siRNA or siRNA targeting Layilin were treated with 1 mg/ml HA35 for 24 h and then challenged with 500 mM ethanol. TEER was measured immediately after the addition of ethanol and 3 h later. Values are expressed as means ± SEM. Values are expressed as means ±SEM, (A) n=3, (B) n=6 and (C) n=4–6, *p < 0.05 compared to cells not treated with ethanol within a transfection group.
Discussion:
Ethanol-induced disruption of intestinal barrier function is an important contributor to the pathophysiological mechanisms for alcohol-induced liver and tissue injury. HA35, a small specific-sized hyaluronan species, has protective effects on intestinal homeostasis in both development and disease (Hill et al., 2013, Kim et al., 2017). Importantly, oral provision of HA35 during short-term ethanol feeding to mice prevented ethanol-induced loss of tight junction integrity in the proximal colon, reduced the concentration of endotoxin in the portal circulation and protected from liver injury (Saikia et al., 2017). Here we investigated the impact of HA35 preventing ethanol-induced loss of co-localization of ZO-1 and occludin along the entire gastrointestinal tract. In contrast to the relatively broad effects of chronic ethanol feeding along the entire intestine (Patel et al., 2015), short-term ethanol primarily impacted the tight junctions in the proximal and distal colon. Provision of HA35 during short-term ethanol feeding maintained tight junctions in both the proximal and distal colon. Similarly, culture of Caco-2 cells with HA35 prevented ethanol-induced loss in co-localization in ZO-1 and occludin and maintained normal permeability and TEER, indicating that HA35 may have direct protective effects on the intestinal epithelium. When expression of the hyaluronan receptor Layilin was knocked-down with targeted siRNA, the protective effects of HA35 were lost. Together these studies identified a role for Layilin in the HA35-mediated preservation of tight junctions in response to ethanol challenge and highlight the potential therapeutic value of HA35 in ameliorating ethanol-induced gut permeability.
In humans with alcohol use disorder, increases in circulating endotoxin are indicative of impaired barrier function (Purohit et al., 2008). Even a single drink of alcohol will increase circulating endotoxin within 24 h (Bala et al., 2014) and increased intestinal permeability can persist for weeks even with complete abstinence from alcohol (Bjarnason et al., 1984), contributing to infection, inflammation and liver injury (Bujanda, 2000). In animal models, ethanol feeding impairs the integrity of tight junctions. The colon is consistently targeted in response to both short term and chronic ethanol feeding (Chaudhry et al., 2016, Cresci et al., 2014, Saikia et al., 2017); however, the region(s) affected by ethanol likely depends on both the dose and duration of ethanol exposure (Patel et al., 2015). Importantly, strategies that restore gut integrity in the colon are associated with a protection from ethanol-induced liver injury, including supplementation with glutamine and tributyrin (Chaudhry et al., 2016, Cresci et al., 2014). Supplementation with zinc (McClain et al., 2017, Mohammad et al., 2012) and supernatants of Lactobacillus GG (Wang et al., 2012) also protect the gut from ethanol-induced injury.
In order to better understand the mechanisms for protection of tight junctions by HA35, here we modeled the in vivo effect of ethanol and HA35 in differentiated Caco-2 cells. Challenge of differentiated Caco-2 monolayers with 40 mM ethanol disrupted the co-localization of ZO-1 and occludin at tight junctions. In particular, there was a significant loss of occludin from the cellular interface in response to ethanol (Figure 3). Occludin is an integral membrane protein critical for suppressing leakage of macromolecules through the epithelial barrier (Al-Sadi et al., 2011). In differentiated Caco-2 monolayers, knockdown of occludin expression increases the permeability of macromolecules (Al-Sadi et al., 2011) and occludin deficient animals develop a weakened gut barrier (Saitou et al., 2000). However, in response to ethanol, the shift of occludin from the cellular interface occurred without a change in total occludin or ZO-1 protein.
Interestingly, functional loss of the transepithelial barrier assessed by TEER was not as sensitive to ethanol exposure as the decreased co-localization of ZO-1 and occludin at the tight junctions and increased permeability to FITC-dextran. Loss of TEER was only observed at higher ethanol concentrations (Figure 3C) and was not affected by inhibition of ethanol metabolism with 4-methylpyrazole (Figure 3D/E). This contrasts to the well recognized impact of acetaldehyde, a product of ethanol metabolism, in disruption of tight junctions in Caco-2 cells observed in other laboratories (Samak et al., 2016). It is possible that other pathways of ethanol metabolism, including catalase or CYP2E1, and/or effects of ethanol that are independent of metabolism contributed to loss of tight junctions. Interestingly, the concentrations of ethanol reported to affect tight junction integrity in Caco-2 cells differs between laboratories. For example, Jonkers laboratory observes loss of tight junction integrity at 40 mM ethanol (Elamin et al., 2014b, Elamin et al., 2014a), while the Anania lab only observes loss of TEER at 3% (513 mM) ethanol (Chopyk et al., 2017), similar to our data (Figure 3C). These differences between laboratories may be due to the use of different clonal variants of the Caco-2 cell line.
Treatment of the Caco-2 cells with HA35 prior to ethanol challenge prevented the ethanol-induced loss of occludin and ZO-1 at the tight junction interface and preserved epithelial barrier function. This protective effect of HA35 on tight junction integrity in the cultured Caco-2 cells indicates that the protective effect of HA35 in a murine model of short-term ethanol exposure is likely due, at least in part, to a direct effect on the intestinal epithelium. A direct protective effect of HA35 with intestinal epithelial cells is consistent with the direct effects of HA35 on expression of tight junction proteins in a murine intestinal organoid system (Kim et al., 2018). However, HA35 may also be acting to protect the gut from ethanol in vivo via indirect effects in suppressing intestinal inflammation (Chen et al., 2015), as HA35 treatment reduces TLR4-mediated signaling in hepatic macrophages (Saikia, et al., 2017).
HA35 required Layilin expression to preserve the localization of tight junction proteins and preserve TEER with ethanol challenge. Layilin is a C-type lectin with putative roles in cell adhesion, motility, and regulation of tight junction permeability (Arpin et al., 2011, Bono et al., 2005, Borowsky and Hynes, 1998). Layilin is highly expressed in epithelial tissue and integrates intracellular signaling mechanisms involved in the maintenance of tight junctions (Adachi et al., 2015, Arpin et al., 2011, Bono et al., 2005). Further, HA35-induced increases in the expression of the tight junction protein ZO-1 are mediated via Layilin both in vivo and in intestinal organoid cultures in vitro (Kim et al., 2018). Interestingly, ethanol exposure disrupted tight junctions independently of changes in the total expression of ZO-1 and occludin (Figure 4B), suggesting that, in contrast to the response of organoids and in vivo mouse experiments, the protective effects of Layilin receptor activity in Caco-2 cells were independent of regulation of ZO-1 expression. This difference may be due to a shorter treatment time for the Caco-2 cells (24 h) compared to the in vivo response (4 days) or differences in the regulation of tight junction proteins between isolated cells and the more structured environment of an organoid or in vivo. These data suggest that, under the conditions of our assay system, both ethanol and HA35 regulate tight junction protein activity and/or localization independent of changes in protein expression. While knockdown of Layilin did not disrupt the localization of ZO-1 or occludin at the paracellular junction in Caco-2 cells under control conditions, expression of Layilin was required for HA35-dependent preservation of tight junctions and TEER during ethanol challenge, indicating a receptor-dependent mechanism of action for HA35 on Caco-2 cells.
In summary, treatment of differentiated human colonic epithelial Caco-2 cells with HA35 was sufficient to preserve the co-localization of ZO-1 and occludin at the tight junction and maintain transepithelial resistance. These data are consistent with a potential direct effect of HA35 via Layilin in preserving tight junctions in the proximal and distal colon during short term ethanol feeding. Taken together, these data suggest that supplementation with HA35 may be a potential therapeutic agent to protect from intestinal barrier dysfunction in patients with ALD.
Grant support:
This work was supported in part by NIH grants: P50 AA024333, R21 AA024387 (LEN); R01AA026764 (LEN and CdlM); Programs of Excellence in Glycosciences Grant HL107147 (CdlM); F30AA026167 (JW) and F31 AA024017 (DB). This work was supported in part by the Case Western Reserve University/Cleveland Clinic CTSA UL1RR024989 and utilized the Leica SP5 confocal/multiphoton microscope that was purchased with partial funding from National Institutes of Health SIG grant 1S10RR026820.
Abbreviations:
- ALD
Alcoholic liver disease
- HA
hyaluronan
- 4-MP
4-methylpyrazole
- PAMP
pathogen associated molecular pattern
- DAMP
danger associated molecular pattern
- ZO-1
zonula occludins-1
- TNFα
tumor necrosis factor α
- MCP-1
monocyte chemoattractant protein-1
- IL6
interleukin 6
- RHAMM
receptor for hyaluronan mediated motility
- TEER
transepithelial electrical resistance
Footnotes
Disclosures:
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- Adachi T, Arito M, Suematsu N, Kamijo-Ikemori A, Omoteyama K, Sato T, Kurokawa MS, Okamoto K, Kimura K, Shibagaki Y, Kato T (2015) Roles of layilin in TNF-alpha-induced epithelial-mesenchymal transformation of renal tubular epithelial cells. Biochemical and biophysical research communications 467:63–69. [DOI] [PubMed] [Google Scholar]
- Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M, Ma T (2011) Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. American journal of physiology. Gastrointestinal and liver physiology 300:G1054–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpin M, Chirivino D, Naba A, Zwaenepoel I (2011) Emerging role for ERM proteins in cell adhesion and migration. Cell adhesion & migration 5:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala S, Marcos M, Gattu A, Catalano D, Szabo G (2014) Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PloS one 9:e96864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I, Ward K, Peters TJ (1984) The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet 1:179–182. [DOI] [PubMed] [Google Scholar]
- Bono P, Cordero E, Johnson K, Borowsky M, Ramesh V, Jacks T, Hynes RO (2005) Layilin, a cell surface hyaluronan receptor, interacts with merlin and radixin. Experimental cell research 308:177–187. [DOI] [PubMed] [Google Scholar]
- Borowsky ML, Hynes RO (1998) Layilin, a novel talin-binding transmembrane protein homologous with C-type lectins, is localized in membrane ruffles. The Journal of cell biology 143:429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujanda L (2000) The effects of alcohol consumption upon the gastrointestinal tract. Am.J.Gastroenterol. 95:3374–3382. [DOI] [PubMed] [Google Scholar]
- Chaudhry KK, Shukla PK, Mir H, Manda B, Gangwar R, Yadav N, McMullen M, Nagy LE, Rao R (2016) Glutamine supplementation attenuates ethanol-induced disruption of apical junctional complexes in colonic epithelium and ameliorates gut barrier dysfunction and fatty liver in mice. The Journal of nutritional biochemistry 27:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Miyamoto Y, Mazagova M, Lee KC, Eckmann L, Schnabl B (2016) Microbiota and Alcoholic Liver Disease. Alcoholism, clinical and experimental research 40:1791–1792. [DOI] [PubMed] [Google Scholar]
- Chen P, Starkel P, Turner JR, Ho SB, Schnabl B (2015) Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology (Baltimore, Md.) 61:883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopyk DM, Kumar P, Raeman R, Liu Y, Smith T, Anania FA (2017) Dysregulation of junctional adhesion molecule-A contributes to ethanol-induced barrier disruption in intestinal epithelial cell monolayers. Physiological reports 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresci GA, Bush K, Nagy LE (2014) Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcoholism, clinical and experimental research 38:1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyphert JM, Trempus CS, Garantziotis S (2015) Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. International journal of cell biology 2015:563818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Motte CA (2011) Hyaluronan in intestinal homeostasis and inflammation: implications for fibrosis. American journal of physiology. Gastrointestinal and liver physiology 301:G945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Motte CA, Kessler SP (2015) The role of hyaluronan in innate defense responses of the intestine. International journal of cell biology 2015:481301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamin E, Masclee A, Dekker J, Jonkers D (2014a) Ethanol disrupts intestinal epithelial tight junction integrity through intracellular calcium-mediated Rho/ROCK activation. American journal of physiology. Gastrointestinal and liver physiology 306:G677–685. [DOI] [PubMed] [Google Scholar]
- Elamin E, Masclee A, Troost F, Pieters HJ, Keszthelyi D, Aleksa K, Dekker J, Jonkers D (2014b) Ethanol impairs intestinal barrier function in humans through mitogen activated protein kinase signaling: a combined in vivo and in vitro approach. PloS one 9:e107421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster C (2008) Tight junctions and the modulation of barrier function in disease. Histochemistry and cell biology 130:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forteza RM, Casalino-Matsuda SM, Falcon NS, Valencia Gattas M, Monzon ME. (2012) Hyaluronan and layilin mediate loss of airway epithelial barrier function induced by cigarette smoke by decreasing E-cadherin. Journal of Biological Chemistry 287:42288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Ahmad MF, Nagy LE, Tsukamoto H (2019) Inflammatory pathways in alcoholic steatohepatitis. Journal of hepatology 70:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirguis J, Chhatwal J, Dasarathy J, Rivas J, McMichael D, Nagy LE, McCullough AJ, Dasarathy S (2015) Clinical impact of alcohol-related cirrhosis in the next decade: estimates based on current epidemiological trends in the United States. Alcoholism, clinical and experimental research 39:2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DR, Rho HK, Kessler SP, Amin R, Homer CR, McDonald C, Cowman MK, de la Motte CA (2013) Human milk hyaluronan enhances innate defense of the intestinal epithelium. The Journal of biological chemistry 288:29090–29104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler SP, Obery DR, Nickerson KP, Petrey AC, McDonald C, de la Motte CA. (2018) Multifunctional role of 35 kDa hyaluronan in promoting defense of intestinal epithelium. J Histochem Cytochem. 66:273–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kessler SP, Obery DR, Homer CR, McDonald C, de la Motte CA (2017) Hyaluronan 35kDa treatment protects mice from Citrobacter rodentium infection and induces epithelial tight junction protein ZO-1 in vivo. Matrix biology : journal of the International Society for Matrix Biology 62:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, West GA, Ray G, Kessler SP, Petrey AC, Fiocchi C, McDonald C, Longworth MS, Nagy LE, de la Motte CA (2018) Layilin is critical for mediating hyaluronan 35kDa-induced intestinal epithelial tight junction protein ZO-1 in vitro and in vivo. Matrix biology : journal of the International Society for Matrix Biology 66:93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain C, Vatsalya V, Cave M (2017) Role of Zinc in the Development/Progression of Alcoholic Liver Disease. Current treatment options in gastroenterology 15:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ (2012) Zinc and liver disease. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition 27:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Behara R, Swanson GR, Forsyth CB, Voigt RM, Keshavarzian A (2015) Alcohol and the Intestine. Biomolecules 5:2573–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J (1991) Alcohol and the small intestine. Scandinavian journal of gastroenterology 26:3–15. [DOI] [PubMed] [Google Scholar]
- Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR (2008) Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol (Fayetteville, N.Y.) 42:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C (2008) Systematic review: glucocorticosteroids for alcoholic hepatitis--a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Alimentary pharmacology & therapeutics 27:1167–1178. [DOI] [PubMed] [Google Scholar]
- Rezaee F, Meednu N, Emo JA, Saatian B, Chapman TJ, Naydenov NG, De Benedetto A, Beck LA, Ivanov AI, Georas SN (2011) Polyinosinic:polycytidylic acid induces protein kinase D-dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells. The Journal of allergy and clinical immunology 128:1216–1224.e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia P, Roychowdhury S, Bellos D, Pollard KA, McMullen MR, McCullough RL, McCullough AJ, Gholam P, de la Motte C, Nagy LE (2017) Hyaluronic acid 35 normalizes TLR4 signaling in Kupffer cells from ethanol-fed rats via regulation of microRNA291b and its target Tollip. Scientific reports 7:15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S (2000) Complex phenotype of mice lacking occludin, a component of tight junction strands. Molecular biology of the cell 11:4131–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samak G, Gangwar R, Meena AS, Rao RG, Shukla PK, Manda B, Narayanan D, Jaggar JH, Rao R (2016) Calcium Channels and Oxidative Stress Mediate a Synergistic Disruption of Tight Junctions by Ethanol and Acetaldehyde in Caco-2 Cell Monolayers. Scientific reports 6:38899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nature methods 9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L (2012) Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Annals of the New York Academy of Sciences 1258:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla PK, Chaudhry KK, Mir H, Gangwar R, Yadav N, Manda B, Meena AS, Rao R (2016) Chronic ethanol feeding promotes azoxymethane and dextran sulfate sodium-induced colonic tumorigenesis potentially by enhancing mucosal inflammation. BMC cancer 16:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W (2012) Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. American journal of physiology. Gastrointestinal and liver physiology 303:G32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]