Abstract
Along many decades, protected environments were targeted by the scientific community for ecological research and for the collection of scientific information related to environmental aspects and biodiversity. However, most of the territory in hotspot regions with weak or even non legal protection has been left aside. These non-protected areas (NPA) could host high biodiversity values. This paper addresses how scientific effort on a NPA (CIAR) of 700 ha from the Atlantic Rain Forest, generates new information and tools for large-scale environmental and biodiversity management in NPAs. Information published during the last decade was summarized and complemented with subsequent novel data about biodiversity (new species, first records, DNA and chemical analyses, etc.). The results showed: 1 new genus (arachnid), 6 new species and several putative new species (fish and arthropod), 6 vulnerable species (bird and mammal) and 36 first records for Argentina (fish, arthropod, platyhelminth and fungi). When compared with protected natural areas of the same biome, the CIAR showed highly valuable aspects for fauna and environment conservation, positioning this NPA as a worldwide hotspot for some taxa. Indeed, when compared to international hotspots in a coordinated Malaise trap program, the CIAR showed 8,651 different barcode index numbers (∼species) of arthropods, 80% of which had not been previously barcoded. Molecules like Inoscavin A, with antifungal activity against phytopathogens, was isolated for the first time in Phellinus merrillii fungi. The study of major threats derived from anthropic activities measured 20 trace elements, 18 pesticides (i.e. endosulfans, chlorpyrifos, DDTs, HCHs) and 27 pharmaceuticals and drugs (i.e. benzoylecgonine and norfluoxetine) in different biotic and abiotic matrices (water, sediment, fish and air biomonitors). This integrated data analysis shows that biodiversity research in NPA is being undervalued and how multidisciplinary and multi-taxa surveys creates a new arena for research and a pathway towards sustainable development in emerging countries with biodiversity hotspots.
Keywords: Environmental sciences, Ecology, Conservation, Monitoring, Hotspot, Pesticides, Agrochemical, Legal protection
1. Introduction
Habitat loss and environmental pollution are currently the major causes for the world-wide decline of biodiversity (Brook et al., 2008; Serengil et al., 2011). Particularly, habitat fragmentation, conversion, and overexploitation are the leading drivers of population decline and species extinctions in tropical hotspots (Myers et al., 2000). In the last decades, a large number of protected areas have been created as an attempt to conserve different vulnerable hotspots (Gray et al., 2016). However, protected areas commonly represent a small proportion of the ecosystems they intend to protect (Myers et al., 2000) and many times, research efforts are only focused on them (Oldekop et al., 2016), leaving aside non-protected areas (NPAs), sometimes more likely to disappear.
The Atlantic Rain Forest (ARF) in South America originally covered around 1,500,000 km2, nevertheless, currently less than 8% of the original cover remains, principally as small fragments (<50 ha) that are isolated from each other (Ribeiro et al., 2009).
More than 110 million people live across the ARF, including colonists of European descent and several Mbyá-Guaraní indigenous groups (∼135,000) (Galindo-Leal et al., 2003; Ribeiro et al., 2011). Forest is commonly replaced by agriculture and livestock pasture, silvopastoral systems and rural areas at a deforestation rate of 20,000 ha/year (INPE, 2015), favoring the loss of biodiversity (extinction of endangered species or those not yet discovered by science) and ecosystem services (Ribeiro et al., 2011).
Despite its significant loss of surface area, the ARF harbours around 7% of the World's flora and fauna (Galindo-Leal et al., 2003) and is one of the 35 most important biodiversity hotspots of the World (Mittermeier et al., 2004). Moreover, it is among the main hotspots with the largest number of endemic plants (>8,000) and vertebrates (567), amounting to 4.8% of total endemic species of the planet (Mittermeier et al., 2004; Myers et al., 2000). More than 110 animal species are officially listed as threatened by International Union for Conservation of Nature (IUCN) and more than 30 as critically endangered (Galindo-Leal et al., 2003), although these numbers may have risen in the last 15 years. In relation to the plants, it was suggested that around 18% of Bromeliaceae and Cactaceae (epiphyte families) have high levels of extinction risk (Leão et al., 2014). Regarding birds, the ARF is the area with most endangered species in the Neotropics (Jenkins et al., 2013).
Although the amount of research on biodiversity and structure of the ARF has increased continuously in the last four decades, it would take approximately 100 years to complete the study of at least 1% of this ecoregion (de Lima et al., 2015). This is especially worrisome because, as other biodiversity hotspots, most of the research efforts are carried out in protected areas (i.e. National parks) (Ferro et al., 2014; Lemes et al., 2014; Loyola et al., 2014; Massara et al., 2015; Paschoal et al., 2016), being the unprotected semi-degraded areas significantly less studied. Moreover, Gray et al. (2016) have suggested that rarefaction-based richness and endemicity often do not differ significantly among unprotected and protected areas. In the ARF there are more than 650 protected areas, with a total surface area of approximately 14,000 km2 (only 1% of the original area) (Galindo-Leal et al., 2003). In this sense, considering the high rate of deforestation and the relatively low sampling effort, the NPAs could disappear before being studied, loosing potential scientific, environmental and cultural richness.
Due to their relative small area and proximity to human settlements, the NPAs are also prone to be affected by several threats. Anthropic environmental pollution proves to be one of the most concerning human effects for the quality of the environment and biodiversity conservation. Pollution of the environmental matrix promotes the degradation of ecosystems, loss of biodiversity, and the impoverishment of ecosystem services that sustain the quality of life of people (Bradshaw et al., 2010; Butchart et al., 2010; Ehrlich and Pringle, 2008). Several studies suggest that the continuous degradation of environments coupled with the growing human population will cause a considerable decrease in biodiversity and quality of human life in the near future (Bradshaw et al., 2010; Butchart et al., 2010; Colombo and Joly, 2010; Serengil et al., 2011). In the last decades, pollutants like heavy metals (Farias et al., 2007; Freire et al., 2012; Kuhlmann et al., 2014), fecal coliform (Casatti et al., 2006; Kuhlmann et al., 2014), and agrochemicals including persistent organic pollutants (De Armas et al., 2007; Freire et al., 2012; Meire et al., 2012; Quinete et al., 2011), have been found in several matrices (fish, water, sediment, soil, air) of the ARF.
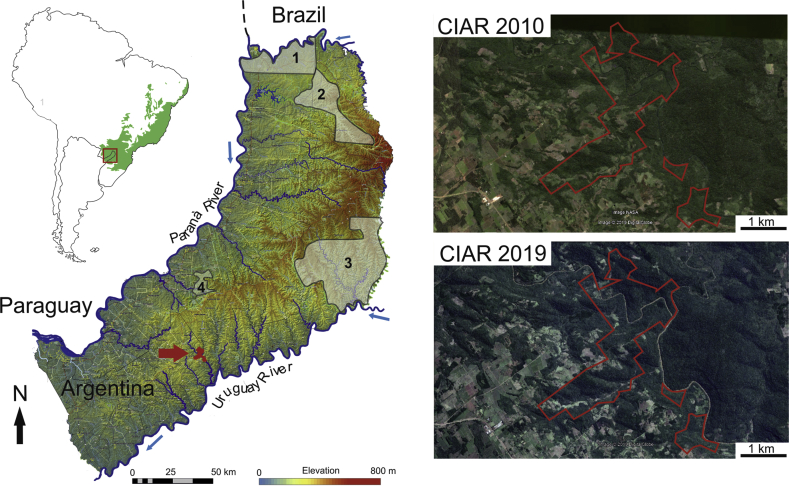
In Argentina, Misiones province concentrates the largest and better preserved relict of a highly diverse biome, which represents the southwestern edge of the ARF (Fig. 1). Most of our knowledge of Misiones biodiversity comes from studies and campaigns carried out in the northwestern extreme of the Province, where large protected areas as Iguazú National Park, Urugua-í, Yabotí, and Salto Encantado provincial reserves (Fig. 1) are located. However, there are various reasons to believe that the study of biodiversity in NPAs is necessary and relevant, which appears to be particularly true for some taxa like lepidopterans, fish, birds and arthropods. Noteworthy, many species do not include protected areas within their natural geographic distribution. Particularly for birds, it has been shown that this is the case for almost 40% of threatened birds at global scale (Rodrigues et al., 2004).
Fig. 1.
Non-protected semi-natural area studied under a restoration program, CIAR (red arrow). ARF, Atlantic Rain Forest; 1, Iguazú National Park; 2, Urugua-í reserve; 3, Yabotí reserve, and 4, Salto Encantado Park. Satellite images show in increment of the vegetal coverage from 2010 to 2019. Map data: Google, DigitalGlobe.
In the presence of such amount of evidence about the importance of non-protected areas for a better knowledge of actual biodiversity on Earth and their exposure to human activities, more efforts should be directed to properly survey these areas. It could be anticipated therefore, that unprotected semi-degraded areas from ARF could be very valuable for the study and conservation of Neotropical biodiversity. If this were the case, biological research efforts should not be concentrated only in protected areas. To this end, the aim of this paper was to assess the biological relevance of an unprotected semi-degraded area, considering the southern extreme of the ARF as a pilot site, in order to encourage research and provide further tools for sustainable management of biodiversity and environment conservation in NPAs. For this reason, we have reviewed and intensified the research efforts in several taxa such as mammals, birds, arthropods (i.e. spiders, butterflies), fish and fungi, for 10 years in a semi-degraded area under a restoration process, highlighting the relative importance of these environments. In order to detect potential threats for the inventoried biodiversity, bacteriological, pharmaceutical and agrochemicals analyses were conducted in both biotic and abiotic matrixes.
2. Materials and methods
2.1. Study area
The ARF is distributed between latitudes 5° and 29° S, including both tropical and subtropical regions (Fig. 1). Currently, a few large fragments remain in locations where geological characteristics make human occupation difficult in protected areas (Ribeiro et al., 2009; Silva et al., 2007). The last largest areas (>10,000 km2) are distributed in two main regions: Serras do Mar (Brazil) and Paranaense forest (Argentina and Brazil), which still include complete species assemblages (Galindo-Leal et al., 2003).
During the last decade, we conducted research in a 700-ha study area in Misiones Province, Argentina. The study area consists of semi-degraded Paranaense secondary forest furrowed by a large number of mountain streams (e.g. Ramos Stream) and the Acaraguá and Del Medio rivers. The study area represents the southern section of a semi-degraded patch of 5,000 ha in the ARF (one of the largest austral patches from Argentina), in which wood has been intensively harvested during the last 50 years. Selective deforestation (extraction of wood, livestock) has affected 85% of the study area, while the remaining 15% was totally deforested. In this area, selective deforestation (extraction of wood, livestock) of a Paranaense secondary forest has been carried out by 85%. Total deforestation was carried out in the remaining 15%. Outside the study area there is no forest, but agricultural crops. In the last half century, tobacco (Nicotiana tabacum), tea (Camellia sinensis) and yerba mate (Ilex paraguariensis) have been cultivated (De Armas et al., 2007; Freire et al., 2012) in the surrounding farmlands. During the last decade around 135,000 seedlings of 50 tree species have been planted in the framework of a biodiversity restoration project. These species were identified as highly impacted (low density) or absent, in accordance with the surveys carried out by local people and in nearby protected areas. Species such as Apuleia leiocarpa, Handroanthus albus, Handroanthus heptaphyllus, Myrocarpus frondosus, Nectandra megapotamica, Cedrela fissilis, Parapiptadenia rigida and Inga uruguensis were extremely rare or absent in the area since 1950. According to the time of reforestation (10 years), the greatest impact occurred in the shrub layers where only pioneer species such as Cecropia pachystachya, Schefflera morototoni, Solanum granuloso-leprosum, Urera baccifera and Trema micrantha were present. Pioneer plants create the conditions of shade and humidity necessary for the survival of reintroduced plants. Moreover, due to the plantations of the first years, the intermediate stratum (10–20 m) is recovering, being this evident in the satellite image analysis (Fig. 1). In the most conserved patches of forest, where the canopy stratum (20–30 m) and some few emergent trees (>30 m) were present, the herbaceous and shrub layer was managed to guarantee access to the light of the seedlings. After 10 years of work, plant cover increased 38%, at an average rate of 3.8% per year (Fig. 1). In terms of legal framework, the study area does not have a strict restriction such as a national park or other protected natural areas. Moreover, the area is not included as part of the green corridor of the province. The main legal instrument over the study area related to land use is the National Law on Minimum Standards for Environmental Protection of Native Forests (National Forest Law: 26,331). This reglamentation promotes the enrichment, restoration, conservation and rational and sustainable use of native forests and their ecosystem services.
2.2. Sampling
With the objective of promoting research in the study area, a biological station (Centro de Investigaciones Antonia Ramos, CIAR) was created by Bosques Nativos Argentinos para la Biodiversidad Fundation in the heart of the semi-degraded 700 ha patch, including accommodation for 16 researchers, a laboratory and terrestrial and aquatic vehicles. A scientific management of the area was part of the foundational objectives to validate the effort and outcomes. In this context, several researchers from different disciplines oriented to the study of biodiversity (birds, arthropods, fishes, mammals and fungi) and environmental pollution have been invited since 2010. Scientists have carried out their research with the logistical support of CIAR and in many cases have published their results in indexed journals. Herein, information published in the last 10 years was summarized and complemented with subsequent unpublished data (new species and records, species checklists, endemisms, chemical analyses, etc.) for the study area.
2.2.1. Mammals
As an approach to assess the presence of mammals, six trap cameras (Moultrie M-990) were employed for three years from 2011 to 2013. The cameras were mounted on traces of animals, small paths and streams, being rotated every 2 months. In some cases corn, salt, sugar cane, and fish were used as bait. Mammal species were identified on the pictures using the guide proposed by Canevari and Vaccaro (2007). Trap cameras were placed opportunistically with the aim of recording the presence or absence of species. This method is not suitable for the estimation of parameters such as abundance and occupancy.
2.2.2. Birds
The inventory of bird species derived from visual observations made during repeated visits to the study area throughout the last decade. In addition, in February 2012 and 2013 birds were mist netted and blood samples for genetic studies were collected. Mist netting was performed by convenience. Blood samples were taken either from the radial or the carotid vessels, and the specimen was photographed and released immediately. All samples were deposited at the National Ultrafrozen Tissue Collection at the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN). A fraction of these samples was used for obtaining DNA barcodes using standardized protocols (Lijtmaer et al., 2012). DNA barcodes are short and standardized sequences of a part of the mitochondrial COI gene that allow the identification of species (Hebert et al., 2003), and these were obtained in the context of a collaboration between Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) and the Centre for Biodiversity Genomics (Canada) for building the reference library of barcode sequences of the birds of the southern cone of South America. In addition to this, we used some of the sequences (including those of a few additional markers such as Cyt b) for specific studies, including the analysis of the taxonomic position of several bird species and various studies of avian evolution.
2.2.3. Fish
Fish species information summarized in this review was gathered from Rosso et al. (2013) and complemented with subsequent unpublished new records for the study area. Some of these new records derived from field surveys whereas others were the result of further taxonomic revisions of voucher material from fish collections (deposited in the Fish Collection of the Instituto de Investigaciones Marinas y Costeras, Unviersidad Nacional de Mar del Plata, Argentina). In addition, DNA barcode analyses also contributed to recognize the actual diversity of the study area.
The field-oriented new records were obtained by the same fishing gears used in Rosso et al. (2013) and complemented with hand-netting. Hand-netting was specifically directed to explore restricted areas not covered by trammel and seine nets in the previous surveys. In order to do so, field surveys were conducted during several visits in April 2012, December 2014, May and October 2016 and April 2017. Further revision of original material collected in the CIAR (deposited in UNMDP) was guided by new species descriptions and systematic revisions from the literature. Formerly unidentified juveniles lacking diagnostic characters were subjected to DNA Barcoding analyses according to Mabragaña et al. (2011). Molecular analysis was also applied to adult specimens in order to test for hidden diversity within otherwise well-defined species.
2.2.4. Arthropods
With over 1 million species formally named, arthropods constitute by far the most diverse class of animals, representing more than half of the described species on Earth (Stork, 2018; Zhang, 2013). This makes arthropods, principally insects, excellent target to assess biodiversity at large taxonomic scales. Moreover, employing passive collecting techniques such as Malaise trapping enables easily and inexpensively obtaining large numbers of specimens for biodiversity studies. The analysis of such a species group does, however, represent a challenge, particularly in terms of identifying the massive amount of specimens that can be collected. This potential problem can be overcame by coupling Malaise trapping with DNA barcoding, thus providing fast, reliable biodiversity assessments at massive scales (deWaard et al., 2018). As a result, the Global Malaise Trap Program (GMP) was launched in 2012 with the objective of studying insect diversity at a global scale and to perform comparative studies among regions. The program includes over 60 traps deployed around the World (Centre for Biodiversity Genomics, CBG (2017). In this context, we deployed two Malaise traps in 2013, which continuously collected flying insects for 2.5 years (February 2013–August 2015). Sample bottles containing 96% ethanol were changed weekly and taken to the MACN. There, bottles were preserved at -20 °C until processing. The materials collected by one of the traps during its first 52 weeks of activity were already processed, barcoded and analyzed. For doing this, the bottles were taken by BB to the CBG at the University of Guelph in 2014 and processed there following the GMP analytical protocol (deWaard et al., 2018). Both the specimens and the DNA extracts returned to MACN after their analysis and are deposited at the National Entomology Collection and the National Ultrafrozen Tissue Collection, respectively.
In addition, Butterflies were manually collected in three samplings instances; October 2012, February 2013 and February and March 2019 by using entomological nets and suspended traps using baits (fruit and sugar solutions). Specimens were preserved in envelopes.
Spiders were also collected in May and June 2011 with four different techniques: beating vegetation, Berlese funnels, cryptic searching, and night collecting. Pseudoscorpions, mites and palpigradi were collected using Berlese funnels in January 2014. Collected specimens were preserved in ethanol 80% for morphological studies.
All the specimens and tissue samples were deposited at MACN.
2.2.5. Fungi
Fungal species were opportunistically collected during 3 years in different seasons (June, September and December 2012; June, November and January 2013 and July, September and March 2014) and identified according to Grassi et al. (2016).
The fungal material was extracted with ethanol and then with ethyl acetate producing an organic extract. The extract of Phellinus merrillii was chromatographed, using mainly reversed-phase, yielding pure compounds and a mixture of hydroxylated fatty acid derivatives, all of which were structurally elucidated by Nuclear Magnetic Resonance and High Resolution Mass Spectrometry. Mass spectrometric analyses were performed using a Bruker MicrOTOF-Q II spectrometer, equipped with electrospray and APCI ion sources. Direct bioautography on TLC, as described previously (Homans and Fuchs, 1970), was employed as the method for detecting fungitoxic substances.
2.2.6. Environment pollution
Data about trace elements and coliform bacteria in water and about pharmaceuticals and drugs in water and fish tissues were taken from Avigliano and Schenone (2016, 2015a) and Ondarza et al. (2019).
In addition, stream water, bottom sediments and plant samples were obtained in the Ramos stream (November 2014), inside the CIAR, in order to monitor the transport of 18 agrochemicals (HCHs, chlordanes, DDTs, endosulfans, chlorpyrifos, trifluraline and chlorothalonil, Table 3) to the study area. Subsurface water samples were collected in pre-cleaned amber glass bottles (N = 3) at 15–20 cm depth, and transported to the laboratory, stored at 4 °C and analyzed within five days after sampling. Bottom sediments (n = 5) were taken using steel core samplers (6 × 10 cm) and kept in aluminum foil at -20 °C until analysis. Mousses and leaves (epiphytic plants) of Tillandsia bergeri (Bromeliaceae) were sampled. Pesticides were analyzed in leaves taken at two different sampling times: t0 background levels (November 2014), and t1 30 months later (May 2016), in order to evaluate the pesticide bioaccumulation in T. bergeri. The leaves were cleaned with a dry brush, wrapped in aluminum foil and kept at -20 °C until analysis. Extraction and clean up procedures of water samples were conducted according to (Gonzalez et al., 2012) while, bottom sediments and epiphytic plants analytes were extracted according to Miglioranza et al. (2003). Samples were analyzed by triplicate by gas chromatography, using a Shimadzu 17-A chromatograph equipped with a 63Ni Electron Capture Detector (GC-ECD) and a SPB-5 [(5%phenyl)-methyl polysiloxane, 30 m × 0.25 mm i. d. × 0.25 μm film thickness, Supelco Inc.] capillary column (Miglioranza et al., 2003). Solvents (pesticide residue analysis grade) and reagents were purchased from Merck Inc. (Germany). Standard solutions of pesticides and PCB-103 (internal standard) were used (Absolute Standards, Ultra Scientific and Sigma Aldrich, USA). Instrumental detection limits ranged between 0.3 to 1.3 ng/g for HCHs (a-, b-, c-, d-), chlordanes (a-, g-, trans-nonachlor), DDTs (pp'-DDE, pp'-DDD, DDT), endosulfans (a-, b-, endosulfan sulfate), chlorpyrifos, trifluraline and chlorothalonil.
Table 3.
Pesticide concentrations in surface water (ng/L) and bottom sediments (ng/g dry wet) from the Ramos Creek (mean ± standard deviation).
| Surface water | Bottom sediments | |
|---|---|---|
| Trifluralin | 0.0002 ± 0.00016 | 0.0002 ± 0.00001 |
| Chlorothalonil | 0.0033 ± 0.0015 | 0.00064 ± 0.00064 |
| Chlorpyriphos | 0.30 ± 0.14 | 0.74 ± 0.13 |
| α-HCH | 0.14 ± 0.07 | 0.05 ± 0.02 |
| γ-HCH | 0.08 ± 0.04 | <dl |
| ƩHCHs | 0.23 ± 0.04 | 0.05 ± 0.02 |
| Heptachlor | <dl | 0.09 ± 0.04 |
| Hept. epoxide | 0.07 ± 0.03 | <dl |
| ƩHeptachlors | 0.07 ± 0.03 | 0.09 ± 0.04 |
| γ-chlordane | 0.08 ± 0.04 | 0.07 ± 0.01 |
| α-chlordane | 0.03 ± 0.01 | <dl |
| ƩChlordanes | 0.11 ± 0.03 | 0.07 ± 0.01 |
| α-endosulfan | 3.71 ± 1.63 | 0.08 ± 0.01 |
| β-endosulfan | 0.36 ± 0.17 | <dl |
| ƩEndosulfans | 4.07 ± 2.37 | 0.08 ± 0.01 |
| pp´-DDE | 0.16 ± 0.08 | <dl |
| pp´-DDD | 0.35 ± 0.16 | <dl |
| ƩDDTs | 0.51 ± 0.13 | <dl |
dl: detection limit.
3. Results and discussion
3.1. Mammals
Trap cameras recorded 19 species of mammals. Among the most common for the region were the Capuchin monkey (Sapajus nigritus), the Overa weasel (Didelphis albiventris), the Big armadillo (Dasypus novemcinctus), the Red brocket deer (Mazama americana) and the little Red brocket deer (M. nana). M. nana is categorized as globally vulnerable since 2015 because its populations had a reduction of 30% in the last 15 years caused mainly by edge effects in the forest remnants, hunting, predation by domestic dogs and diseases acquired from domestic ungulates (Duarte et al., 2015).
The rodents Hydrochoerus hydrochaeris (Capibara), Cuniculus paca (Paca), Dasyprocta azarae (Agutí bayo), Myocastor coypus (Coipo), Cavia aperea (Cuís), Sciurus aestuans (Gray squirrel) and Sylvilagus brasiliensis (Tapetí) were recorded.
The carnivores observed were the Crab-eating fox (Cerdocyon thous), Coatí (Nasua nasua), Crab-eating raccoon or Aguará Popé (Procyon cancrivorus), Neotropical otter (Lontra longicaudis), Lesser grison (Galictis cujua), and felines such as Leopardus pardalis (Ocelot) (Fig. 2) and L. tigrinus (Tirica). L. pardalis has been classified as vulnerable for Argentina (Aprile et al., 2012), while L. tigrinus was categorized as globally vulnerable by the IUCN (Payan and Oliveira, 2016). In the Argentine ARF, it is estimated that the L. pardalis population has been reduced by more than 50% in the last 20 years, with a current estimated population of between 600 and 800 individuals (Aprile et al., 2012; Di Bitetti et al., 2008). Estimates suggest a population of less than 1,000 individuals in the entire Atlantic Forest (Payan and Oliveira, 2016). However, it has been reported that density of L. tigrinus in degraded environments could increase due to decreased competition with L. pardalis, which population is reduced by hunting and loss of habitat (Oliveira et al., 2010).
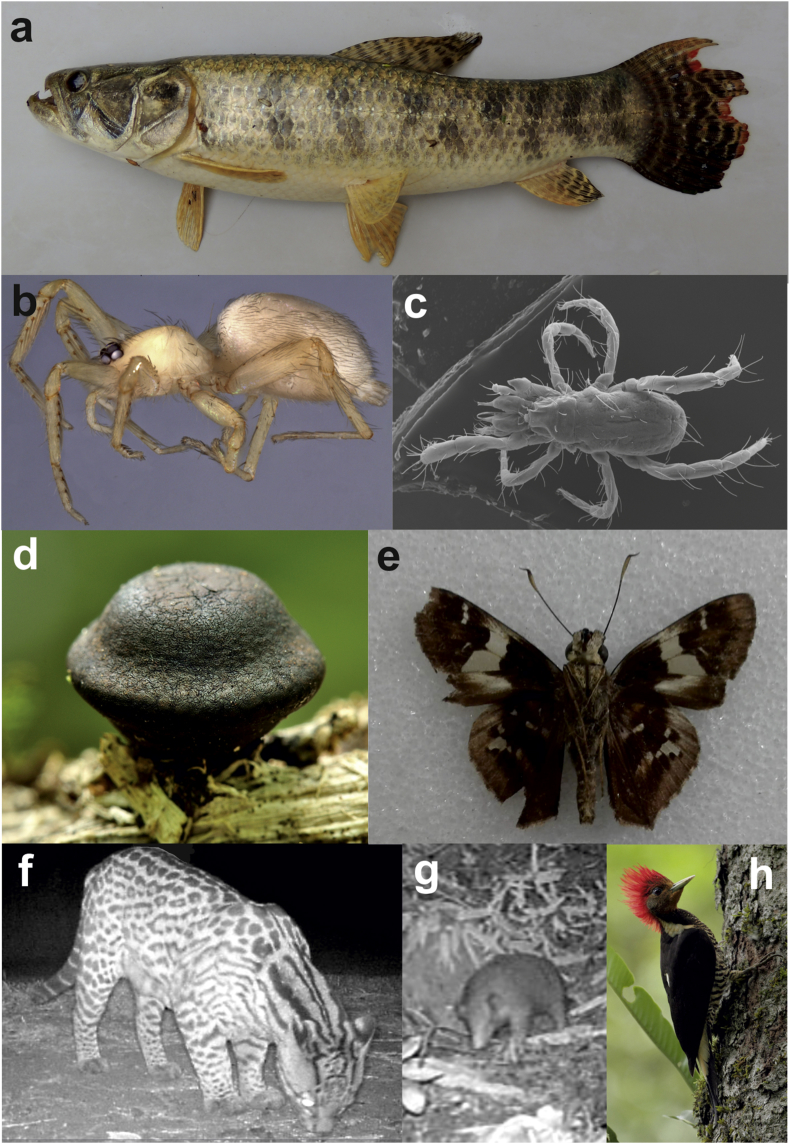
Fig. 2.
Representation of some new species, first records and vulnerable species found at CIAR. New species: Hoplias misionera (a), Neotrops poguazu (b), Poeciliophysis pratensis (c); first record: Phylacia turbinate (d), Vacerra bonfilius (e); vulnerable species: Leopardus pardalis (f); Cabassous tatouay (g), Celeus galeatus (h). The pictures b and h were yielded by C. Grismado and J. M. Lammertink, respectively.
Other species classified as vulnerable for Argentina (Aprile et al., 2012) such as the Cabasú (Cabassous tatouay) and the Tamandua (Tamandua tetradactyla) were also observed in the CIAR.
The major carnivores like the jaguar (Panthera onca) and the cougar (Puma concolor) and herbivorous such as the tapir (Tapirus terrestris) and peccaries (Tayassu sp) have not been found, which highlights the highly degradaded habitat and the hunting pressure in the region. These species inhabit, with a high level of threat, the Salto Encantado provincial park (70 km north of the study area) (Cirignoli et al., 2011), which is not connected to the studied forest mass. In other parks located further north (Iguazú, Yabotí and Urugua-í parks) (Fig. 1), these species are more abundant, although in a vulnerable or endangered situation (Massoia et al., 2012; Paviolo et al., 2016). Nevertheless, 5 threatened mammal species have been recorded in the study area, marking the relative importance of the non-protected degraded environments for mammal conservation.
3.2. Birds
The total number of species detected in the bird observation trips and the two field campaigns was 95 (Table S1). It is worth mentioning that this detected diversity is by no means complete and it only reflects the richness observed in occasional field trips. In particular, the high abundance of the Red-ruffed Fruitcrow (Pyroderus scutatus) is remarkable and should be highlighted. This is one of the largest passerine species in the World, characterized by a very low frequency song. This species also has a lek inside the CIAR, which was observed both in 2012 and 2013 and is presumably still active (leks are a widespread form of mating system among cotingids).
Of the 95 species detected, 94 already have DNA barcodes in the Barcode of Life Data Systems (BOLD; Ratnasingham and Hebert (2007)) website (see Table S1). These sequences are contributing to the large-scale study of avian diversity in the southern cone of South America. The initial stages of this study (Kerr et al., 2009; Lijtmaer et al., 2011) included 500 species (50% of Argentine avian richness) and the samples collected at CIAR are part of the second stage of the analysis.
In addition to this large-scale study, some of the samples collected at CIAR were also included in other, more specific studies. A particularly relevant case is that of the Helmeted Woodpecker (Celeus galeatus) (Fig. 2). This species is globally threatened (like others in the genus Celeus) and its distribution has been important in Argentina to define "Important Bird and Biodiversity Areas" (IBAs; Di Giacomo (2005)). Its presence in CIAR thus confirms the relevance of this area for bird conservation (and for conservation in general) and therefore the importance of studying its biodiversity. Moreover, genetic analyses that included the sample collected at CIAR confirmed that this species, previously considered to belong to the genus Dryocopus, actually belongs to the genus Celeus. In fact, this analysis is one of the studies that contributed to the current agreement among specialists in placing the species in the genus Celeus. Finally, this species is also interesting from a behavioral perspective because it represents a case of social dominance mimetism (Prum, 2014): its plumage pattern strongly resembles that of a sympatric and larger species, the Robust Woodpecker (Campephylus robustus).
Another study that we are currently undertaking and includes samples collected at CIAR is that of the House Wren (Troglodytes aedon), the most widely distributed passerine of the American Continent. These samples are contributing with the evolutionary history analyses of this species, which is confirming the previously detected presence of several mitochondrial lineages in this species (Campagna et al., 2012; Kerr et al., 2009), allowing to establish with further confidence that the Malvinas form (Troglodytes cobbi) is a different species and enabling a much more detailed study of the diversification history of the species complex and the role of glacial cycles in its southern Neotropical distribution.
Finally, samples from a few of the species collected at CIAR were also used in a project in which our research group is studying the evolutionary history of forest Neotropical birds. In particular, samples from the Black-goggled Tanager (Trichothraupis melanops), the Buff-browed Foliage-gleaner (Syndactyla rufosuperciliata) and the Mottle-cheeked Tyrannulet (Phylloscartes ventralis) have been used to analyze the history of the cycles of connection and isolation between the Atlantic Forest and the Andean tropical forests (Yungas) and their effects on the Neotropical avifauna (Cabanne et al., 2019; Trujillo-Arias et al., 2018). These studies, considered together with other analyses of avian species present in the Atlantic Forest and the Andean forests (i.e. Trujillo-Arias et al., 2017) indicate that the response of birds to the cycles of connection and isolation of these forests is idiosyncratic and depends on the dispersion abilities and other ecological attributes of each species. As a consequence, the level of gene flow between the populations of each species in the ARF and the Andean forests differ among species, as does the area of contact between populations. In some of them, in fact, the populations of the ARF (which are sometimes currently considered different subspecies) have been shown to deserve species status, which would add to the long list of birds that are endemic to this forest (i.e. Habia rubica, Syndactyla rufosupercilliata, Ramphotrigon megacephalum).
A recent study by Wu et al. (2018) concluded that as a consequence of climate change the composition of birds in National Parks (using US as a model) is drastically changing: they expect a turnover higher than 20% for 2050, with some new species arriving to the National Parks and others not longer present in them. This highlights the dynamism of species distributions in the face of climate change and the need for the study of different areas in each ecoregion and not only the protected ones.
3.3. Fish
Combining previous findings (Rosso et al., 2013) with revision of voucher material, new field samplings and DNA barcoding analyses resulted in a total of 46 fish species currently known for CIAR (Table S2). From the original species list (n = 23), nomenclature of 10 species was modified (re-diagnosis or new combination) whereas 12 taxa remained unmodified. Only one species was removed. Twenty three new records were added, representing an increase of 100% in fish species richness of the study area.
Field samplings yielded 17 new records for CIAR, covering 3 orders and 9 families. Four families, Anostomidae, Prochilodontidae, Bryconidae and Auchenipteridae are new for the original species list. DNA Barcoding allowed the recognition of Oligosarcus jenynsii, Bryconamericus iheringii and Astyanax sp. from unidentified juveniles collected during original samplings in 2011. Molecular analyses also revealed the existence of likely cryptic species within Salminus brasiliensis (Rosso et al., 2018) and Astyanax saguazu. With the revision of voucher material in fish collections, two new records (Crenicichla minuano and Rineloricaria catamarcensis) were registered. Revision of voucher material also allowed removing Cyanocharax (Diapoma) lepiclastus from the original species list. Three putative new undescribed species were also identified. A re-diagnosis of the miniature catfish formerly identified as Microglanis cottoides showed a unique combination of characters for the genus. Similarly, Rineloricaria sp. remained undiagnosed and also housed a unique combination of characters within known species of the genus. The new record of Ancistrus sp. also likely represents a new taxon.
Ichthyological exploration of this small semi-natural area reveals a hardly anticipated large amount of new evidence about species diversity. For instance, CIAR contains the type locality of the recently described Hoplias misionera (Rosso et al., 2016) (Fig. 2) and also the first records for Argentina (Rosso et al., 2013) of the giant blue thraira Hoplias lacerdae and the small characin Bryconamericus maromba. The molecular characterization of type material of H. misionera collected in the CIAR was included in a continental-wide approach (Cardoso et al., 2018) to unravel genetic divergence within the H. malabaricus species complex. In this scenario, H. misionera represented the basal taxon in the H. malabaricus clade. The Gymnogeophagus sp. reported by (Rosso et al., 2013), could be now diagnosed as Gymnogeophagus lipokarenos, an unknown species until 2015 (Malabarba et al., 2015). Astyanax xiru, a common and abundant species in field surveys of the CIAR during the last years (Avigliano et al., 2018), was described (De Lucena et al., 2013) and first reported for Argentina (Casciotta et al., 2016) just recently. Interestingly, the number of Astyanax species (n = 9) collected in freshwater ecosystems of the CIAR, almost matched the entire specific richness (n = 10) of this genus in the Middle Uruguay River. Molecular operational units recovered for the genus Astyanax in the CIAR contributed to a comprehensive analysis of the genetic diversity and divergence of the genus in South America (Rossini et al., 2016).
When compared with a large protected natural area of the same biogeographic region, CIAR still presents valuable aspects for fish fauna conservation (Table 1). In spite of its notorious smaller area, ichthyological surveys in the CIAR reported just a slightly lower number of species but a rather similar number of fish families. Moreover, the contribution of this small unprotected area to the alpha diversity of the Uruguay River drainage is relevant, with a new species, three new records and three putative undescribed new species (Table 1).
Table 1.
Administrative and biological aspects of selected large protected (Yabotí) and small Non-protected semi-natural area (CIAR) in the middle Uruguay River drainage (see Fig. 1).
| Yabotí | CIAR | ||
|---|---|---|---|
| Attributes | |||
| Area (ha) | 221,155 | 700 | |
| Year of creation | 1993 | 2010 | |
| Fish assemblages | |||
| Orders | 4 | 4 | |
| Families | 16 | 14 | |
| Species | 65 | 46 | |
| New species (a) | 6 | 1 (c) | |
| New records (b) | 2 | 3 | |
| (a) Species with type material collected in the natural area | |||
| Oligosarcus amome | Hoplias misionera | ||
| Astyanax paris | |||
| Astyanax ojiara | |||
| Hisonotus aky | |||
| Bryconamericus uporas | |||
| Australoheros ykeregua | |||
| (b) Species firstly recorded for Argentina | Leporinus amae | Bryconamericus maromba | |
| Astyanax xiru | Hoplias lacerdae | ||
| Rhamdella longiuscula | |||
| (c) Putative new undescribed species | Rineloricaria sp. | ||
| Microglanis sp. | |||
| Ancistrus sp. | |||
Overall, our results highlight the relevance of continuous sampling and multidisciplinary approach for the identification of fish species in freshwater ecosystems of small non-protected semi-natural areas of the ARF. Both aspects are crucial for an accurate estimation of alpha diversity, a central task in conservation. Indeed, fishes recently gave an example of how a more exhaustive survey of non-protected areas furnishes new evidence of hidden diversity. The description of a new very peculiar family of fishes inhabiting near-urban semi-natural areas in the Amazonia (De Pinna et al., 2017) is a vast example of the relevance of non-protected areas for a proper knowledge of actual biodiversity.
3.4. Arthropods
3.4.1. Malaise traps
In total, more than 75,000 terrestrial arthropods were collected throughout the first year of one of the traps. Sequences were successfully obtained from almost 68,000 of them (90% success). In total, 8,651 different Barcode Index Numbers (BINs, which are a close proxy for species; Ratnasingham and Hebert (2007)) were collected, 80% of which had not been previously barcoded and were therefore new for the World's barcode database. This striking biodiversity becomes even more evident if one considers that this trap collected more specimens and more BINs (i.e. species) than any other trap of the GMP in the world, including several placed in protected areas (such as National Parks). The relevance of assessing species diversity in such a biodiverse and understudied area is stressed by the fact that this trap is also the one that added more unique BINs (i.e. species that were only collected in this site and are therefore present in the BOLD database only as a result of the trap deployed at CIAR; Centre for Biodiversity Genomics 2017).
Regarding the most abundant Orders, Diptera led the list followed by Hemiptera, Lepidoptera, Hymenoptera and Coleoptera. Dipterans usually are the most collected insects in Malaise traps, with hymenopterans also among the richest groups (deWaard et al., 2018; Geiger et al., 2016). Consistently (Stork, 2018), mentioned that focus needs to be placed in some of these groups (Diptera, Hymenoptera, Coleoptera) because they are very speciose and therefore include several understudied families.
It is relevant to consider that more than 4,000 BINs were singletons in the Malaise trap (i.e. only one specimen was collected in the entire year). This suggests that one trap operating in one year cannot collect all the diversity of insects of such a biodiverse area and that for sure there is an even higher insect richness at CIAR than the one detected.
Ecological analyses aimed at assessing the main factors responsible for the variation of abundance of the most common BINs are underway. Preliminary results highlight the high variation among BINs in the patterns of seasonal abundance, contrasting with more temperate areas in which most species are more abundant in the same moment of the year (Geiger et al., 2016). A recent review of global insect richness estimations (Stork, 2018) stressed that to advance in the knowledge of diversity it is particularly relevant to perform extensive sampling, particularly in Neotropical areas that have been understudied, and to use rapid DNA techniques that could help to better estimate the effect of cryptic species. If one takes these recommendations into consideration, assessing insect diversity in the ARF at a large taxonomic scale and using a DNA-based identification technique (i.e. DNA barcoding) is clearly relevant.
3.4.2. Butterflies
The total number of species of diurnal butterflies found in the CIAR was 340 (Table S3). The Hesperiidae family showed the highest richness with 146 species, followed by Nymphalidae (118), Lycaenidae (25), Riodinidae (22), Pieridae (17) and Papilionidae (12). The most sampled genera (with more than 5 species) were: Adelpha (Nymphalidae) with 9 species, Dynamine (Nymphalidae) with 7, and Urbanus (Hesperiidae) with 6. Four first records have been reported for Argentina (Kolana ligurina, Antigonus minor, Vacerra bonfilius and Lychnuchus celsus) (Fig. 2) and for Misiones province (Dardarina daridaeus) (first record for both the genus and species) (Núñez Bustos, 2017, 2016). Some species that are scarce in other areas from ARF forest, like Elkalyce cogina (Lycaenidae), Narope panniculus (Nymphalidae) and Heliopetes purgia (Hesperiidae) (Núñez Bustos, 2016), have also been found. Moreover, considering the species found in the semi-degraded border areas located less than 4 km from CIAR, which were explored with more intensity during the last 12 years, the list of species increases from 346 to 500, adding 3 first records for Argentina (Synargis ethelinda, Archaeoprepona amphimachus y Euphyes fumata) (Núñez Bustos, 2016). With around 900 species, the Misiones province is the area with the greatest diversity of butterflies in Argentina (Avigliano and Schenone, 2015b; Núñez Bustos et al., 2013). The 500 species found in the CIAR and surroundings represent 70% of the species found in the Iguazú National Park (N = ∼700) (Núñez Bustos, 2009), 83% in comparison with the Yacutinga Reserve (N = ∼600, Misiones) (Núñez Bustos, 2008), and 40 and 25 % of the species recorded in Argentina and the whole ARF (N = ∼2000) (Iserhard et al., 2017), respectively, indicating that the CIAR and its surroundings are a butterfly hotspot for the entire ecoregion. In addition, Campo Ramón locality (CIAR and its surroundings forest) was indicated as one of the most important butterfly hotspots for Argentina (Klimaitis et al., 2018).
3.4.3. Spiders
As a result of the survey 85 species were collected (Table S4) distributed on 69 genera of 23 families. A new genus (Fig. 2) and four new species of the family Oonopidae were described (Grismado and Ramírez, 2013), three of them with CIAR as type locality: Neotrops piacentinii, N. rubioi, and N. poguazu. As far as we know, these three species have been found only at this area, then, new records may determine if they are endemic. Instead, N. pombero is widely distributed from Paraguay to the Argentinian provinces of Misiones, Corrientes and Buenos Aires as the southern limit. Other specimens belonging to an undescribed genus (temporarily placed in Oonops) were also found. Seven first records were reported for Argentina (Table S4): Alpaida alto an Araneidae previously known from the department of Alto Paraná in Paraguay (Levi, 1988); Enoploctenus cyclothorax a Ctenidae widely distributed in Brazil (WSP, 2019); Sphecozone novaeteutoniae a Linyphiidae only known from few specimens from Santa Catarina state of Brazil (Baert, 1987); Hogna gumia, a Lycosidae described by Tullgren (WSP, 2019) based on material from Bolivia; Caayguara cupepemassu a Sparassidae wild distributed in Southern Brazil (Rheims, 2010); Chrysometa boraceia a Tetragnathidae from southeastern Brazil (Levi, 1986); and the Theridiidae Thwaitesia affinis known from Panamá to Paraguay (WSP, 2019).
3.4.4. Other arthropods
The order Palpigradi was reported for the first time in northeastern Argentina with Eukoenenia (Eukoeneniidae) which don't belong to any cosmopolitan species of the order previously recorded for Argentina (Harvey, 2013): E. florenciae and E. hanseni.
Representatives of the order Pseudoscorpions included an interesting ensemble of two groups of species which belong to the southern extreme of the distribution of one neotropical genus or to the northern “gondwanic” one. In the former group Pseudochthonius aff. brasiliensis (Chthoniidae) and Ideoroncus cf. palidus (Ideoroncidae), both are the first records for the genus in Argentina. In the other group the genus Austrochthonius (Chthoniidae) is represented by at least two species: A. paraguayensis and a putative new species that is currently being described. Other first records for Argentine were Lechytia chthoniiformis (Lechythiidae), Paratemnoides nidificator (Atemmnidae) and Progarypus sp. (Olpiidae).
The suborder Prostigmata (order Trombidiformes) was highly diverse at CIAR. As an example, a putative new species of the “gondwanic” genus Sellnickiella represented the first record of the family Labidostommatidae in Argentina (species not yet formally described). The family Rhagidiidae was well represented (Porta and Bertrand, 2016) with Poeciliophysis (Dentocheles) pratensis, Robustocheles (R.) mucronata and a putative new species of the subgenus Foveacheles (Mediostella), four first records for South America.
Porta (2016) has reported the first record of the subfamily Newportiinae (Chilopoda: Scolopendromorpha: Scolopocryptopidae) and the species Newportia balzanii from Argentina.
In relation to Coleoptera, Libonatti et al. (2018) have described for the first time the morphology and chaetotaxy of the last instar larva and the pupa of Ora depressa (Scirtidae), providing diagnostic characters and information on its biology. Field studies of Canthon quinquemaculatus (Scarabaeidae) has revealed a new food relocation behavior, providing new observations on preferential directions of rolling in Scarabaeinae (Cantil et al., 2019). Moreover, Dinghi (2013) has reported the first case of brood chambers provisioned exclusively with leaf litter, mostly of fumo bravo (Solanum granuloso), in a dung beetle (Dichotomius carbonarius) (Scarabaeidae).
3.5. Fungi
A total of 137 fungal species were found, distributed in 13 orders, 32 families and 86 genera. Several species can have different notorious uses such as medicinal, food and handicrafts (pipes) (Table 2).
Table 2.
Most notorious Fungal species found and their putative used.
| Order | Species | Uses |
|---|---|---|
| Pezizales | Rickiella edulis | Edible |
| Agaricales | Lycoperdon sp. | Edible |
| Agaricales | Marasmius crinis-equi | Ecological association with Cacicus haemorrhous and Cacicus chrysopterus |
| Agaricales | Macrolepiota sp. | Edible |
| Agaricales | Oudemansiella platensis | Edible |
| Agaricales | Pleurotus albidus | Edible |
| Agaricales | Ciclocybe cylindracea | Edible |
| Agaricales | Psilocybe cubensis | Medicinal |
| Agaricales | Schizophyllum commune | Medicinal and Edible |
| Auriculariales | Auricularia fuscosuccinea | Edible |
| Auriculariales | Auricularia nigricans | Edible |
| Auriculariales | Auricularia delicata | Edible |
| Phallales | Phallus indusiatus | Edible |
| Polyporales | Ganoderma applanatum | Medicinal |
| Polyporales | Ganoderma lucidum | Medicinal |
| Polyporales | Hydnopolyporus fimbriatus | Edible |
| Polyporales | Rigidoporus ulmarius | Medicinal |
| Polyporales | Favolus tenuiculus | Edible |
| Polyporales | Fomes fasciatus | Cultural use (pedernal) |
| Polyporales | Lentinus velutinus | Cultural use (traditional Guaraní pipes) |
| Polyporales | Lentinus swartzii | Edible |
| Polyporales | Polyporus spp. | Edible |
| Polyporales | Pycnoporus sanguineus | Cultural use (nNatural colourant) |
| Polyporales | Trametes versicolor | Medicinal |
The complete checklist of fungal species found was published by Grassi et al. (2016), being the sole publication of fungal diversity in Misiones province in areas outside national protected areas. More than 37% correspond to the Polyporaceae family. This figure is important because the presence and abundance of many Polyporoid fungi are associating with the diversity of different host trees and a good management of dead deciduous trees, stumps, logging residues, and leaf litter. Studies based in the physiology of the fungi found in CIAR show forest management applied there, in which debris and logs are not removed from the environment, plays an important role in the establishment of saprophyte species that contribute to the decaying process and nutrient recycling (Grassi, 2017).
The trend to include NPAs in study programs is increasing (Gray et al., 2016). Such international trend is revealing the importance of conservation and restoration of native ecosystems, because natural populations of fungi are not always represented in protected areas. For example, the Iguazú National Park (Fig. 1) has been well characterised over the years and 375 fungal species were found over a decade of sampling (Wright and Wright, 2005). However, among species found in CIAR, Phylacia turbinata is the first report for Argentina (Grassi et al., 2016) (Fig. 2), while Rickiella edulis, a species with a very ephemeral fruiting body, was found for the first time in Misiones province, and it was just the third record for Argentina (Vignale et al., 2015). In addition, Sir et al. (2018) have recorded the first reports of Annulohypoxylon stygium for Northwestern Argentina.
During our screening for antifungal metabolites, wood-inhabiting Phellinus merrillii fungus collected at CIAR was investigated. Inoscavin A was isolated and identified for first time in this paper for P. merrillii. Moreover, ergosteroids like ergosterol, ergosterol peroxide, Δ7,9−11 ergosterol peroxide, isoergosterone and a mixture of ethyl 2-hydroxy-fatty esters (A) were also isolated. The fatty acid had from 20 to 31 carbon atoms, and being the metabolite with 22 carbons atoms the major one in the mixture.
Inoscavin A was previously reported for Inonotus xeranticus, P. baumi and P. igniarius (Kim et al., 1999; Zhu et al., 2016). It is receiving major attention because of its potential anticarcinogenic properties based on its function as natural antioxidant (Zhu et al., 2016). Here, Inoscavin A showed antifungal activity against phytopathogens.
The mixture of the 2-hydroxy fatty esters also displayed good antifungal activity. 2-hydroxy fatty acids are major constituents of fungal sphingolipids (Van Dyk et al., 1994), the acids themselves or their esters are common ingredients in cosmetics, skin creams, and lotions because of their particular physical properties, and the display of a complex monolayer phase behavior. However, 2-hydroxy very long chain fatty acids, as those isolated in this work, are rare, and their biological role is still being investigated (Sassa and Kihara, 2014).
Taking into account these results, the potential use of these metabolites, or the fungus, as medicinal drugs or cosmetics becomes possible.
3.6. Other taxa
Negrete and Brusa (2017) have reported a new record of the land planarian Obama ladislavii (Platyhelminthes: Geoplanidae) for Argentina.
3.7. Pollution
3.7.1. Pesticides
Pesticide levels are reported here for the first time in the study area (CIAR). Concentrations of legacy pesticides (4.5 ng/L) in stream water were several times higher than those of chlorpyrifos, an authorized insecticide (0.3 ng/L). Endosulfan presented the highest concentrations reaching up to 77% of total pesticide levels (4.07 and 5.29 ng/L, respectively, Table 3). Endosulfan was an insecticide widely used for agriculture purposes in horticulture, perennial (yerba mate, tea, tobacco) and extensive crops (soybean) until 2013, when its application was fully banned in our country (SENASA, 2011). Misiones province is the major producer of perennial crops together with citric and forest plantations (Izqulerdo et al., 2008). These productions are supported by severe pesticide application, such as endosulfan, glyphosate, and chlorpyriphos, among others (Gonzalez, 2007). The 10.3 α-/β-endosulfan ratio found in water samples was higher than those found in the technical mixture of this insecticide (α-/β -isomers = 2.33). The heavy rainfall (2000 mm/year) in the ARF could increase the wash out of pesticides (Izqulerdo et al., 2008). Thus, the results found in surface water could be a consequence of an enhanced runoff of aged endosulfan from agricultural soils, facilitated by its relative low soil adsorption and relatively high hydrophilicity (log Kow <4, Sabljić et al., 1995). Similar results reported by Jergentz et al. (2005) showed that storm events produced edge-of-field runoff of technical endosulfan applied in soybean fields from the Pampa region, in Argentina.
Inside the DDT group, only pp´-DDD and pp´-DDE were found (0.35 and 0.16 ng/L, respectively). The observed predominance of both metabolites over the parent compound, suggest a historic source of DDT in the surrounding soils that contributes to the load in the Ramos stream. Furthermore, α- and γ-HCH isomers account for 61% and 39% of the total HCH concentrations, respectively (Table 3).
Considering the Argentinean Aquatic Biota Protection Limits (ABPL) in freshwater environments, pesticide concentrations were below recommended maximum levels SRH 2005).
Bottom sediments showed a different pesticide pattern distribution. Chlorpyrifos concentrations were the highest (0.75 ng/g dry wet, Table 3). Endosulfans, chlordanes and heptachlor (0.08, 0.07 and 0.09 ng/g dry wet, respectively, Table 3) account for 30% each one of the total organochlorine pesticide levels (2.06 ng/g dry wet, Table 3).
The atmosphere represents an important spread way from local to global scale of pesticides (Wania, 2003). Intensive agricultural practices are considered one of the main pesticide sources for the environment through surface runoff, spray drift or volatilization processes. Nowadays, atmospheric biomonitoring with plants allows evaluating pesticide concentrations with different species, such as pine needles, mousses, lichens, and epiphytes (Gonzalez et al., 2010; Ratola et al., 2014). However, leaves of Tilandsia sp. have been shown to be a suitable monitor for organic contaminants (Gonzalez et al., 2010).
Chlorpyrifos presented the highest levels in all epiphytic plants analyzed, which were between 6 and 9 times higher compared to other pesticides (Table 4). Bioaccumulation of this organophosphorus insecticide in leaves of T. bergeri was six times higher than background levels (considering t1-t0 concentrations, Table 4). Moss samples presented only four pesticides (chlorpyrifos, pp'-DDD, α-endosulfan, γ-HCH), which reached similar concentrations as in the leaves of T. bergeri (Table 4).
Table 4.
Pesticide concentrations found in epiphytic plants (ng/g dry wet) from the reserve (mean ± standard deviation).
| Leaves Tillandia bergeri |
Moses | ||||
|---|---|---|---|---|---|
| t0: background | t1: 30 months later | Bioaccumulation (t1--t0) | |||
| Chlorpyriphos | 13.4 ± 3.25 | 19.4 ± 0.48 | 6 | Chlorpyriphos | 29.7 ± 11.6 |
| α-HCH | 0.10 ± 0.05 | 0.21 ± 0.03 | 0.11 | α-HCH | <dl |
| γ-HCH | 0.16 ± 0.06 | 0.17 ± 0.04 | 0.01 | γ-HCH | 1.31 ± 1.40 |
| ƩHCHs | 0.26 ± 0.13 | 0.38 ± 0.02 | 0.16 | ƩHCHs | 1.31 ± 1.40 |
| Heptachlor | 0.08 ± 0.04 | <dl | <dl | <dl | |
| Hept. epoxide | <dl | <dl | <dl | <dl | |
| ƩHeptachlors | 0.08 ± 0.04 | <dl | <dl | ƩHeptachlors | <dl |
| α-chlordane | 0.14 ± 0.07 | 0.21 ± 0.01 | 0.07 | α-chlordane | <dl |
| γ-chlordane | 0.02 ± 0.01 | 0.05 ± 0.09 | 0.03 | γ-chlordane | <dl |
| ƩChlordanes | 0.16 ± 0.11 | 0.26 ± 0.11 | 0.10 | ƩChlordanes | <dl |
| α-endosulfan | 0.25 ± 0.12 | 0.63 ± 0.19 | 0.38 | α-endosulfan | 1.25 ± 0.52 |
| β-endosulfan | <dl | 0.10 ± 0.03 | β-endosulfan | <dl | |
| endosulfan sulfate | 0.10 ± 0.05 | 0.62 ± 0.41 | 0.52 | endosulfan sulfate | <dl |
| ƩEndosulfans | 0.35 ± 0.08 | 1.35 ± 0.30 | 1.0 | ƩEndosulfans | 1.25 ± 0.52 |
| pp´-DDE | 0.08 ± 0.04 | 0.42 ± <dl | 0.34 | pp´DDE | <dl |
| pp´-DDD | 0.66 ± 0.02 | 0.61 ± <dl | -0.05 | pp´-DDD | 0.70 ± 0.80 |
| ƩDDTs | 0.74 ± 0.03 | 1.03 ± 0.14 | 0.29 | ƩDDTs | 0.70 ± 0.80 |
dl: detection limit.
3.7.2. Trace elements, pharmaceuticals, drugs and coliform bacteria
The concentration of trace elements (Ag, Al, As, Ba, Be, Cd, Co, Cr, Cs, Cu, Fe, Ga, Mn, Ni, Pb, Se, Sr, Ti, U, V and Zn), glyphosate and fecal coliform in surface water have been explored in the CIAR (Avigliano et al., 2019; Avigliano and Schenone, 2015a). Based on the Canadian ABPL for freshwater, trace elements were below recommended maximum level.. Fecal coliform (9–930 CFU/100 ml) was above the recommended levels established for consumption and recreational use (USEPA, 2012), and were very close to the recommended limits for crop irrigation (1,000 CFU/100 ml, WHO (1989)) (Avigliano and Schenone, 2015a). These values were lower than in other protected areas of the ARF such as Iguazú National Park (Fig. 1), where levels were higher than 1,000 CFU/100 ml) (Avigliano and Schenone, 2015a). Although glyphosate peaks in surface water were reported in other semi-degraded ARF areas from Argentina and Brazil during storms (Avigliano and Schenone, 2015a; De Armas et al., 2007; Freire et al., 2012), this was not detected in the study area (Avigliano and Schenone, 2015a). Recently, 27 pharmaceuticals, illicit drugs and their metabolites in tissues (muscle, liver and gill) of three native fish species were measured in the study area (values lower than the maximum residue limits for human consumption) (Ondarza et al., 2019). Antibiotics such as erythromycin, sulfamethoxazole, sulfathiazole and trimethoprim were detected, which are used in human medicine, aquaculture and livestock (Ondarza et al., 2019). Moreover, caffeine, norfluoxetine (metabolite of the antidepressant fluoxetine), and benzoylecgonine (metabolite of cocaine) have also been detected in fish tissues (Ondarza et al., 2019).
3.8. Concluding remarks, political and management issues
Large-scale assessments of biodiversity are essential in the context of the increasing threat posed to the World's fauna and flora. These include both studying particular taxonomic groups in extensive geographic regions and quantifying biodiversity at a large taxonomic scale in particular areas. This latter approach is crucial for understanding the relevance of preserving particular biomes, ecoregions or even smaller areas with particular ecological, climatic or geological characteristics. As many other areas with natural resources, the anthropic pressure is the main cause of biodiversity loss in semi-degraded areas. However, as this study clearly shows a wide range of measures are available to assess biodiversity values in non-protected and thus commonly undervalued areas. The lack of a strong legal framework, or the location in a fragmented landscape and outside green corridors, should not be limiting factors but an opportunity to build knowledge in sensitive sectors. This was evident in the NPA surveyed in this study considering the relatively high specific richness in most examined taxa, the new genus (1) and species (5) described, the first records for species (30) and genera found (6), as well as the endangered species reported. Then, it is possible to state that the NPA assessed is a particularly significant location for the study and conservation of the Neotropical biodiversity. As an area representing the non-protected southernmost distribution of the ARF, its study is very relevant for the understanding of the marginal forest biodiversity and its relationship with core areas of this biome. In this regard, it is important to mention that notorious differences in biodiversity composition (especially in fish, birds and arthropods), ecology and evolutionary history have been reported between the northern and southern extensions of the ARF in Northwestern Brazil. Therefore, the study of the diversity and characteristics of these taxa in the southernmost distribution of the ARF in the CIAR would undoubtedly help to understand the characteristics of this complex biome and its biodiversity.
Many of the principal findings of this study are partly due to the concentrated sampling effort conducted in the study area. Therefore, it may be expected that applying the same survey effort to protected areas could produce a similar increase in knowledge. Certainly, statistically comparable sampling programs between protected areas and NPAs are needed to investigate this issue. However, distribution of organisms is not always uniform respect to the extension of sampling, and isolated areas of biodiversity hotspots may flourish at different scales of sampling. This study is a good contribution to this point.
For some taxa such as fish and butterflies, it was possible to compare richness between CIAR (results of this work) and protected non-degraded zones (published data, i.e. Núñez Bustos, 2008, 2009, 2016; Cirignoli et al., 2011; Grassi et al., 2016) nearby (between 70–250 km) such as Iguazú, Yabotí, Urugua-í and Salto Encantado Parks, with similar biogeographic characteristics. Due to the degree of conservation, extension (there is no edge effect) and resources received by large protected areas, it would be possible to expect to find greater diversity in these areas in relation to NPAs. However, for fish and butterflies, our results showed a comparable richness between protected and NPAs. Moreover, our results showed particularities such as new species and records, which could be lost due to the high rate of habitat loss from NPAs. On the other hand, the presence of endangered species found in CIAR highlights its ecological relevance for conservation.
In relation to the conservation of the NPAs, where biodiversity has been reduced by human activity, it is necessary to implement reforestation and restoration projects, green corridors, sustainable production systems and communication programs to maximize the possibilities for long term balance, promoting a sustainable development. It is singularly important because several organisms are associated with specific plants or assemblages, which could have been deteriorated. Historically, traditional medicine has made use of natural remedies since ancient times, and the popular use of fungi and plant extractives has led to the discovery of many pharmaceutical active ingredients (Butler, 2008). Since these secondary metabolites are biosynthesized as a response to the environment, it is important to collect the plants from their natural habitats. As such, major secondary metabolites, which can be obtained in sustainable and environmentally-friendly ways from abundant plant species, can be used as leading compounds for chemical transformation to enhance their pharmaceutical applications and as natural, environmentally-friendly agrochemicals (Loiseleur, 2017). In many cases, their biological activities have been determined as antifeedants, antifungals, toxins, antioxidants, or insecticides, among others (Demain and Fang, 2000). Several secondary metabolites were identified in plants (i.e. kokusaginine, flindersiamine, glutinol, olivacine, uleine) such as Helietta apiculata (Canela de venado), Balfourodendron riedelianum (Guatambú blanco), Aspidosperma australe (Guatambú amarillo) and Cabralea canjerana (Cancharana) (unpublished data) and in Fungi (Inoscavin A in P. merrillii, with demonstrated antifungal activity against F. tucumaniae) collected at CIAR. In this regard, this paper shows that the conservation of semi-natural environments is also valuable for the discovery and development of the medicines of the future.
In addition to the positive and promissory findings of this research, our survey also detected several detrimental aspects from the anthropic component of this semi-degraded NPA of the ARF. Particularly, several pollutants and xenobiotics were detected in different biotic and abiotic matrices. The presence of pollutants is not only worrisome for the conservation of biodiversity, but also for human health, because many people ingest water or fish directly in the area, including indigenous people of the Guarani ethnic group (Avigliano and Schenone, 2016, 2015a) Nevertheless, reforestation projects can contribute to reduce the aquatic pollution because forests can filter out sediment (vegetation reduces ) and contaminants (excess nutrients, agrochemicals) applied to the land (Avigliano and Schenone, 2015a).
The outstanding biodiversity reported here makes a very strong case for claiming stronger approach to biodiversity and the reduction of anthropic damage in non-protected areas near (or within) biodiversity hotspots. For a long time, the large, protected biodiversity hotspots have been considered the appropriate areas to perform biodiversity research. The fact that the application of this paradigm for decades could not stop the evident loss of biodiversity due to anthropic pressure, even after the biodiversity hotspots global policy was applied (Butchart et al., 2010), should be interpreted by the scientific community as a warning to review the research efforts and think about traditional conservation policies. Williams (2011) highlights the aspects that require the most urgent attention, both in terms of conservation and human well-being, which should influence considerably biodiversity research.
Non-protected, semi-degraded areas are exposed to the daily use by humans as part of their economies, culture and recreation. Consistently, local policy-makers take the community values and characteristics into account in the design and encourage of management plans. As stated by Jepson (2001) “Global biodiversity plan needs to convince local policy-makers”. In this context, and taking into account the complexity of achieving conservation objectives, it is clear that there is no single recipe and that an interdisciplinary approach (Pohl and Hirsch Hadorn, 2008) is more necessary than ever (Marchese, 2015).
The present study does not only reveal new ecological data in a semi-degraded area close to a biodiversity hotspot, hopefully encouraging researchers to further study these environments, but also transcend the merely scientific scope by developing the following transdisciplinary topics (among others) to support sustainable development in the region:
-
•
Strengthening the provincial regulations on resources (i.e. fish)
-
•
Involvement of local government in restoration actions
-
•
Development of sustainable small scale cattle models
-
•
Development of forest native restoration models
-
•
Social media communication actions
-
•
Cultural events to promote reforestation actions
-
•
Best management practices by private sector
In summary, the integral biological data analysis of this work under multidisciplinary and multi-taxonomic surveys in a semi-degraded NPA yielded valuable contributions for research and management of these areas. It could be expected that if NPAs are managed responsibly, they certainly will constitute a precious path towards sustainable development in emerging countries with biodiversity hotspots.
Declarations
Author contribution statement
Esteban Avigliano, Nahuel Schenone: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Juan Jose Rosso, Dario Lijtmaer, Paola Ondarza, Luis Norberto Piacentini, Matias Izquierdo, Adriana Cirigliano, Gonzalo Romano, Ezequiel Nuñez Bustos, Andres Porta, Ezequiel Mabragaña, Emanuel Grassi, Jorge Palermo, Belen Bukowski, Pablo L. Tubaro: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors wish to thank to Centro de Investigación Antonia Ramos (CIAR), Fundación Bosques Nativos Argentinos para la Biodiversidad and Via Bariloche for logistical support and Ministerio de Ecología y Recursos Naturales Renovables de la Provincia de Misiones for granting collection permits. We also thank the Secretaría de Ambiente y Desarrollo Sustentable of Argentina for granting export permits for some of the barcoded materials. Some of the field campaigns were possible thanks to grants from CONICET and Agencia Nacional de Promoción Científica y Tecnológica. DNA barcodes were obtained thanks to the Fondo iBOL Argentina and the Centre For Biodiversity Genomics, University of Guelph, Canada. We are also greatly indebted to H. Schenone, J. Unizony, M. Homanchuk and family, G. Ramos, P. Araya and family, C. Ramos and family, Eusebio Soto, E. Rolón, A. Esmoris, L. Diaz Caragli and F. Biolé for their invaluable collaboration in the field. We thank to P. Teta, M. Di Bitetti, P. Cruz, D. Varela and A. Paviolo for their help in identifying mammal species and to G. Cabrera, A. Pato, M. Capra Coarasa for their contributions in fungal metabolites analysis.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Aprile G., Cuyckens E., De Angelo C., Di Bitetti M., Lucherini M., Muzzachiodi N., Palacios R., Paviolo A., Quiroga V., Soler L. 2012. Libro Rojo de Mamíferos Amenazados de la Argentina, Sociedad Argentina para el Estudio de los Mamíferos (SAREM), Argentina. [Google Scholar]
- Avigliano E., Clavijo C., Scarabotti P., Sánchez S., Llamazares Vegh S., del Rosso F.R., Caffetti J.D., Facetti J.F., Domanico A., Volpedo A.V. Exposure to 19 elements via water ingestion and dermal contact in several South American environments (La Plata Basin): from Andes and Atlantic Forest to sea front. Microchem. J. 2019;149:103986. [Google Scholar]
- Avigliano E., Rolón M.E., Rosso J.J., Mabragaña E., Volpedo A.V. Using otolith morphometry for the identification of three sympatric and morphologically similar species of Astyanax from the Atlantic Rain Forest (Argentina) Environ. Biol. Fish. 2018 [Google Scholar]
- Avigliano E., Schenone N.F. Water quality in the Atlantic Rainforest Mountain Rivers (South America): quality indices assessment, nutrients distribution and consumption effect. Environ. Sci. Pollut. Res. 2016 doi: 10.1007/s11356-016-6646-9. [DOI] [PubMed] [Google Scholar]
- Avigliano E., Schenone N.F. Human health risk assessment and environmental distribution of trace elements, glyphosate, fecal coliform and total coliform in Atlantic Rainforest mountain rivers (South America) Microchem. J. 2015;122:149–158. [Google Scholar]
- Avigliano E., Schenone N.F. Nymphalidae, papilionidae and pieridae (insecta: Lepidoptera: rhopalocera) from the Acaraguá river basin, Misiones, Argentina. Entomotropica. 2015;30:84–91. [Google Scholar]
- Baert L. The genus brattia simon, 1894 in South America (araneae Linyphiidae) Bull. en Ann. van K. Belgische. 1987;123:261–265. [Google Scholar]
- Bradshaw C.J.A., Giam X., Sodhi N.S. Evaluating the relative environmental impact of countries. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook B.W., Sodhi N.S., Bradshaw C.J.A. Synergies among extinction drivers under global change. Trends Ecol. Evol. 2008;23:453–460. doi: 10.1016/j.tree.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Butchart S.H.M., Walpole M., Collen B., Van Strien A., Scharlemann J.P.W., Almond R.E.A., Baillie J.E.M., Bomhard B., Brown C., Bruno J., Carpenter K.E., Carr G.M., Chanson J., Chenery A.M., Csirke J., Davidson N.C., Dentener F., Foster M., Galli A., Galloway J.N., Genovesi P., Gregory R.D., Hockings M., Kapos V., Lamarque J.F., Leverington F., Loh J., McGeoch M.A., McRae L., Minasyan A., Morcillo M.H., Oldfield T.E.E., Pauly D., Quader S., Revenga C., Sauer J.R., Skolnik B., Spear D., Stanwell-Smith D., Stuart S.N., Symes A., Tierney M., Tyrrell T.D., Vié J.C., Watson R. Global biodiversity: indicators of recent declines. Science. 2010;328:1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- Butler M.S. Natural products to drugs: natural product-derived compounds in clinical trials. Nat. Prod. Rep. 2008;31:1612–1661. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- Cabanne G.S., Campagna L., Trujillo-Arias N., Naoki K., Gómez I., Miyaki C.Y., Santos F.R., Dantas G.P.M., Aleixo A., Claramunt S., Rocha A., Caparroz R., Lovette I.J., Tubaro P.L. Phylogeographic variation within the buff-browed foliage-gleaner (aves: furnariidae: Syndactyla rufosuperciliata) supports an andean-atlantic forests connection via the cerrado. Mol. Phylogenetics Evol. 2019;133:198–213. doi: 10.1016/j.ympev.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Campagna L., St Clair J.J.H., Lougheed S.C., Woods R.W., Imberti S., Tubaro P.L. Divergence between passerine populations from the Malvinas - Falkland Islands and their continental counterparts: a comparative phylogeographical study. Biol. J. Linn. Soc. 2012;106:865–879. [Google Scholar]
- Canevari M., Vaccaro O. LOLA; Buenos Aires, Argentina: 2007. Guía de Mamíferos del Sur de América del Sur. [Google Scholar]
- Cantil L.F., Sánchez M.V., Dinghi P.A., Genise J.F. Food relocation behavior, nests, and brood balls of Canthon quinquemaculatus laporte de Castelnau (Coleoptera: Scarabaeidae: Scarabaeinae) Coleopt. Bull. 2019;68:199–209. [Google Scholar]
- Cardoso Y.P., Rosso J.J., Mabragaña E., González-Castro M., Delpiani M., Avigliano E., Bogan S., Covain R., Schenone N.F., Díaz de Astarloa J.M. A continental-wide molecular approach unraveling mtDNA diversity and geographic distribution of the Neotropical genus Hoplias. PLoS One. 2018;13 doi: 10.1371/journal.pone.0202024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casatti L., Langeani F., Silva A.M., Castro R.M.C. Stream fish, water and habitat quality in a pasture dominated basin, southeastern Brazil. Braz. J. Biol. 2006;66:681–696. doi: 10.1590/s1519-69842006000400012. [DOI] [PubMed] [Google Scholar]
- Casciotta J., Almirón A., Říčanová Š., Dragová K., Piálek L., Říčan O. First record of Astyanax xiru Lucena, Castro & Bertaco, 2013 (Characiformes: Characidae) from the río Uruguay basin of Argentina. Ichthyol. Contrib. PecesCriollos. 2016;42:1–4. [Google Scholar]
- CBG . 2017. Centre for Biodiversity Genomics [WWW Document]http://biodiversitygenomics.net/resources/ibol-2017/ [Google Scholar]
- Cirignoli S., Galliari C.A., Pardiñas U.F.J., Podestá D.H. Mamíferos de la reserva Valle del Cuña Pirú, Misiones, Argentina. Mastozool. Neotrop. 2011;18:24–43. [Google Scholar]
- Colombo A., Joly C. Brazilian Atlantic Forest lato sensu: the most ancient Brazilian Forest, and a biodiversity hotspot, is highly threatened by climate change. Braz. J. Biol. 2010;70:697–708. doi: 10.1590/s1519-69842010000400002. [DOI] [PubMed] [Google Scholar]
- De Armas E.D., Monteiro R.T.R., Antunes P.M., Dos Santos M.A.P.F., De Camargo P.B., Abakerli R.B. Diagnóstico espácio-temporal da ocorréncia de herbicidas nas águas superficiais e sedimentos do Rio Corumbatá e principais afluentes. Quim. Nova. 2007;30:1119–1127. [Google Scholar]
- de Lima R.A.F., Mori D.P., Pitta G., Melito M.O., Bello C., Magnago L.F., Zwiener V.P., Saraiva D.D., Marques M.C.M., de Oliveira A.A., Prado P.I. How much do we know about the endangered Atlantic Forest? Reviewing nearly 70 years of information on tree community surveys. Biodivers. Conserv. 2015;24:2135–2148. [Google Scholar]
- De Lucena C.A.S., Castro J.B., Bertaco V.A. Three new species of Astyanax from drainages of southern Brazil (Characiformes: Characidae) Neotrop. Ichthyol. 2013;11:537–552. [Google Scholar]
- De Pinna M., Zuanon J., Rapp Py-Daniel L., Petry P. A new family of neotropical freshwater fishes from deep fossorial amazonian habitat, with a reappraisal of morphological characiform phylogeny (Teleostei: Ostariophysi) Zool. J. Linn. Soc. 2017;182:76–106. [Google Scholar]
- Demain A.L., Fang A. History of Modern Biotechnology I. first ed. Springer; Berlin, Heidelberg, Berlin: 2000. The Natural Functions of Secondary Metabolites. [Google Scholar]
- deWaard J.R., Levesque-Beaudin V., DeWaard S.L., Ivanova N.V., McKeown J.T.A., Miskie R., Naik S., Perez K.H.J., Ratnasingham S., Sobel C.N., Sones J.E., Steinke C., Telfer A.C., Young A.D., Young M.R., Zakharov E.V., Hebert P.D.N. Expedited assessment of terrestrial arthropod diversity by coupling Malaise traps with DNA barcoding. Genome. 2018:1–11. doi: 10.1139/gen-2018-0093. [DOI] [PubMed] [Google Scholar]
- Di Bitetti M.S., Paviolo A., De Angelo C.D., Di Blanco Y.E. Local and continental correlates of the abundance of a neotropical cat, the ocelot (Leopardus pardalis) J. Trop. Ecol. 2008;24:189–200. [Google Scholar]
- Di Giacomo A.S. 2005. Important Bird Areas in Argentina. Priority sites for the conservation of biodiversity. Aves Argentinas and Associación Ornitológica del Plata, Buenos Aires. [Google Scholar]
- Dinghi P.A. Leaf-litter brood chambers in Dichotomius (Luederwaldtinia) carbonarius (Mannerheim, 1829) (Coleoptera: Scarabaeidae): a novel behavior for dung beetles. Coleopt. Bull. 2013;67:388–397. [Google Scholar]
- Duarte J.M., Vogliotti A., Cartes J.L., Oliveira M.L. Mazama nana. IUCN red list threat. SPECIES. 2015 T29621A22154379. [Google Scholar]
- Ehrlich P.R., Pringle R.M. Where does biodiversity go from here? A grim business-as-usual forecast and a hopeful portfolio of partial solutions. Proc. Natl. Acad. Sci. 2008;105:11579–11586. doi: 10.1073/pnas.0801911105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias M., Neto J., Lima V., Lira V., Franco E. Riscos sociais e ambientais devido a presença de metais pesados nas águas superficiais no distrito industrial de Mangabeira. Qualis. 2007;6 11–10. [Google Scholar]
- Ferro V.G., Lemes P., Melo A.S., Loyola R. The reduced effectiveness of protected areas under climate change threatens Atlantic Forest tiger moths. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire R., Schneider R.M., de Freitas F.H., Bonifacio C.M., Tavares C.R.G. Monitoring of toxic chemical in the basin of Maringa Stream/Monitoramento de compostos quimicos toxicos na bacia do ribeirao Maringa. Acta Sci. Technol. 2012;34:295–302. [Google Scholar]
- Galindo-Leal C., de Gusmão Cãmara I., Sayre D. The Atlantic Forest of South America: biodiversity status, threats, and outlook. Electron. Green J. 2003 [Google Scholar]
- Geiger M., Moriniere J., Hausmann A., Haszprunar G., Wägele W., Hebert P., Rulik B. Testing the Global Malaise Trap Program – how well does the current barcode reference library identify flying insects in Germany? Biodivers. Data J. 2016;4 doi: 10.3897/BDJ.4.e10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. Región mesopotámica, Provincia de Misiones. In: De Paula E.M., editor. La Problemática de Los Agroquímicos y Sus Envases, Su Incidencia En La Salud de Los Trabajadores, La Población Expuesta y El Ambiente. Estudio Colaborativo Multicéntrico. 2007. pp. 137–180. [Google Scholar]
- Gonzalez M., Miglioranza K.S.B., Shimabukuro V.M., Londoño O.M.Q., Martinez D.E., Aizpún J.E., Moreno V.J. Surface and groundwater pollution by organochlorine compounds in a typical soybean system from the south Pampa, Argentina. Environ. Earth Sci. 2012;65:481–491. [Google Scholar]
- Gonzalez M., Ondarza P.M., Mitton F., Fillmann G., Miglioranza K. 20th an. Meeting Soc. Environ. Toxicol. Chem. Europe. Sevilla, Spain. 2010. Polybrominated Diphenyl Ethers (PBDEs) in the atmosphere of the center- Patagonian region of Argentina: assessment using pine needles and epiphytes. [Google Scholar]
- Grassi E. Universidad de Buenos Aires; 2017. Prospección de cepas nativas del género Polyporus para la producción de enzimas lignocelulolíticas y su potencial aplicación en biopulpado y bioblanqueo. [Google Scholar]
- Grassi E.M., Romano G.M., Schenone N.F. Macrohongos presentes en un área de manejo regenerativo de bosque de Mata Atlántica (Misiones, Argentina) Bol. la Soc. Argentina Bot. 2016;51:223–233. [Google Scholar]
- Gray C.L., Hill S.L.L., Newbold T., Hudson L.N., Boïrger L., Contu S., Hoskins A.J., Ferrier S., Purvis A., Scharlemann J.P.W. Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat. Commun. 2016;7:12306. doi: 10.1038/ncomms12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grismado C.J., Ramírez M.J. The new world goblin spider of the new genus Neotrops (Araneae: Oonopidae), Part I. Bull. Am. Mus. Nat. Hist. 2013;338:1–150. [Google Scholar]
- Harvey M. 2013. Palpigrades of the World [WWW Document]http://www.museum.wa.gov.au/catalogues/palpigrades [Google Scholar]
- Hebert P.D.N., Cywinska A., Ball S.L., DeWaard J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Biol. Sci. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homans A.L., Fuchs A. Direct bioautography on thin-layer chromatograms as a method for detecting fungitoxic substances. J. Chromatogr. A. 1970;51:327–329. doi: 10.1016/s0021-9673(01)96877-3. [DOI] [PubMed] [Google Scholar]
- INPE . Instituto Nacional de Pesquisas Espaciais; 2015. Atlas dos Remanescentes Florestais da Mata Atlântica - período 2013-2014, Fundação SOS Mata Atlântica. [Google Scholar]
- Iserhard C.A., Uehara-Prado M., Marini-Filho O.J., Duarte M., Freitas A.V.L. Fauna da Mata Atlântica: Lepidoptera-Borboletas. In: Monteiro Filho E., Conte C.E., editors. Revisões Em Zoologia: Mata Atlântica. Curitiba. 2017. p. 490. [Google Scholar]
- Izqulerdo A.E., De Angelo C.D., Aide T.M. Thirty years of human demography and land-use change in the Atlantic Forest of Misiones, Argentina: an evaluation of the forest transition model. Ecol. Soc. 2008;13:3. [Google Scholar]
- Jenkins C.N., Pimm S.L., Joppa L.N. Forest dynamics and land-use transitions in the Brazilian Atlantic Forest: the case of sugarcane expansion. Proc. Natl. Acad. Sci. 2013:E2602–E2610. doi: 10.1073/pnas.1302251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson P. Global biodiversity plan needs to convince local policy-makers. Nature. 2001;409 doi: 10.1038/35051132. [DOI] [PubMed] [Google Scholar]
- Jergentz S., Mugni H., Bonetto C., Schulz R. Assessment of insecticide contamination in runoff and stream water of small agricultural streams in the main soybean area of Argentina. Chemosphere. 2005;61:817–826. doi: 10.1016/j.chemosphere.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Kerr K.C.R., Lijtmaer D.A., Barreira A.S., Hebert P.D.N., Tubaro P.L. Probing evolutionary patterns in neotropical birds through DNA barcodes. PLoS One. 2009;4:e4379. doi: 10.1371/journal.pone.0004379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.P., Yun B., Shim Y., G, Y.I. Inoscavin A, a new free radical scavenger from the mushroom Inonotus xeranticus. Tetrahedron Lett. 1999;40:6643–6644. [Google Scholar]
- Klimaitis J., Núñez Bustos E., Klimaitis C., Güller R. Vázquez Mazzini; Buenos Aires: 2018. Mariposas- Butterflies-Argentina. Guía de Identificación. [Google Scholar]
- Kuhlmann M.L., Imbimbo H.R.V., Ogura L.L., Villani J.P., Starzynski R., Robim M. de J. Effects of human activities on rivers located in protected areas of the Atlantic Forest. Acta Limnol. Bras. 2014;26:60–72. [Google Scholar]
- Leão T.C.C., Fonseca C.R., Peres C.A., Tabarelli M. Predicting extinction risk of Brazilian Atlantic Forest angiosperms. Conserv. Biol. 2014;28:1349–1359. doi: 10.1111/cobi.12286. [DOI] [PubMed] [Google Scholar]
- Lemes P., Melo A.S., Loyola R.D. Climate change threatens protected areas of the Atlantic Forest. Biodivers. Conserv. 2014;23:357–368. [Google Scholar]
- Levi H.W. The neotropical orb-weaving spiders of the genus Alpaida (Araneae: Araneidae) Bull. Mus. Comp. Zool. 1988;151:365–487. [Google Scholar]
- Levi H.W. The neotropical orb-weaver genera Chrysometa and Homalometa (Araneae: Tetragnathidae) Bull. Mus. Comp. Zool. 1986;151:91–215. [Google Scholar]
- Libonatti M.L., Jorge G., Archangelsky M., Michat M.C. The last instar larva and pupa of Ora depressa (Coleoptera: Scirtidae), a marsh beetle with underwater pupation. Acta Entomol. Musei Natl. Pragae. 2018;58:441–445. [Google Scholar]
- Lijtmaer D.A., Kerr K.C.R., Barreira A.S., Hebert P.D.N., Tubaro P.L. DNA barcode libraries provide insight into continental patterns of avian diversification. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijtmaer D.A., Kerr K.C.R., Stoeckle M.Y., Tubaro P.L. Methods in Molecular Biology. Humana Press; Totowa: 2012. DNA barcoding birds: from field collection to data analysis. [DOI] [PubMed] [Google Scholar]
- Loiseleur O. Natural products in the discovery of agrochemicals. Chim. Int. J. Chem. 2017;71:810–822. doi: 10.2533/chimia.2017.810. [DOI] [PubMed] [Google Scholar]
- Loyola R.D., Lemes P., Brum F.T., Provete D.B., Duarte L.D.S. Clade-specific consequences of climate change to amphibians in Atlantic Forest protected areas. Ecography. 2014;37:65–72. [Google Scholar]
- Mabragaña E., de Astarloa J.M.D., Hanner R., Zhang J., Castro M. DNA barcoding identifies argentine fishes from marine and brackish waters. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malabarba L.R., Malabarba M.C., Reis R.E. Descriptions of five new species of the Neotropical cichlid genus Gymnogeophagus Miranda Ribeiro, 1918 (Teleostei: Cichliformes) from the rio Uruguay drainage. Neotrop. Ichthyol. 2015;13:637–662. [Google Scholar]
- Marchese C. Biodiversity hotspots: a shortcut for a more complicated concept. Glob. Ecol. Conserv. 2015;3:297–309. [Google Scholar]
- Massara R.L., De Oliveira Paschoal A.M., Doherty P.F., Hirsch A., Chiarello A.G. Ocelot population status in protected Brazilian Atlantic Forest. PLoS One. 2015;10 doi: 10.1371/journal.pone.0141333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoia E., Chebez J., Bosso A. Fundación Feliz de Azara; Buenos Aires: 2012. Los mamiferos silvestres de la provincia de Misiones, Argentina. [Google Scholar]
- Meire R.O., Lee S.C., Targino A.C., Torres J.P.M., Harner T. Air concentrations and transport of persistent organic pollutants (POPs) in mountains of southeast and southern Brazil. Atmos. Pollut. Res. 2012;3:417–425. [Google Scholar]
- Miglioranza K.S.B., Aizpún De Moreno J.E., Moreno V.J. Dynamics of organochlorine pesticides in soils from a southeastern region of Argentina. Environ. Toxicol. Chem. 2003;22:712–717. [PubMed] [Google Scholar]
- Mittermeier R., Gil P., Hoffman M., Pilgrim J., Brooks T., Mittermeier C., Lamoreux J., Fonseca G. 2004. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Ecoregions, Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Ecoregions. Cemex, Sierra Madre. [Google Scholar]
- Myers N., Mittermeler R.A., Mittermeler C.G., Da Fonseca G.A.B., Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Negrete L., Brusa F. Increasing diversity of land planarians (Platyhelminthes: Geoplanidae) in the interior Atlantic Forest with the description of two new species and new records from Argentina. Zootaxa. 2017;4662:99–127. doi: 10.11646/zootaxa.4362.1.5. [DOI] [PubMed] [Google Scholar]
- Núñez Bustos E. Registros inéditos de mariposas diurnas (Lepidoptera: Papilionoidea) para Argentina III. Colección Núñez Bustos en el MACN. Trop. Lepid. Res. 2017;27:78–85. [Google Scholar]
- Núñez Bustos E. Nuevos registros de Lepidoptera para Argentina (Lepidoptera: Papilionoidea & Bombycoidea) SHILAP. 2016;44:645–651. [Google Scholar]
- Núñez Bustos E. Mariposas diurnas (Lepidoptera: Papilionoidea y Hesperioidea) del Parque Nacional Iguazú, Provincia de Misiones, Argentina. Trop. Lepid. Res. 2009;19:71–81. [Google Scholar]
- Núñez Bustos E. Diversidad de mariposas diurnas en la Reserva Privada Yacutinga, Provincia de Misiones, Argentina (Lepidoptera: Hesperioidea y Papilionoidea) Trop. Lepid. Res. 2008;18:92–101. [Google Scholar]
- Núñez Bustos E., Dalí H., Zapata L. Misiones Mariposas - Butterflies - Borboletas, Golden Com. 2013. Buenos Aires. [Google Scholar]
- Oldekop J.A., Holmes G., Harris W.E., Evans K.L. A global assessment of the social and conservation outcomes of protected areas. Conserv. Biol. 2016;30:133–141. doi: 10.1111/cobi.12568. [DOI] [PubMed] [Google Scholar]
- Oliveira T.G., Tortato M.A., Silveira L., Kasper C.B., Mazim F.D., Lucherini M., Jácomo A.T., Soares J.B.G., Marques R.V., Sunquist M. The Biology and Conservation of Wild Felid. Oxford University; Oxford: 2010. Ocelot ecology and its effect on the smallfelid guild in the lowland neotropics; pp. 559–580. [Google Scholar]
- Ondarza P.M., Haddad S.P., Avigliano E., Miglioranza Karina S.B., Brooks B.W. Pharmaceuticals, illicit drugs and their metabolites in fish from Argentina: implications for protected areas influenced by urbanization. Sci. Total Environ. 2019;649:1029–1037. doi: 10.1016/j.scitotenv.2018.08.383. [DOI] [PubMed] [Google Scholar]
- Paschoal A.M.O., Massara R.L., Bailey L.L., Kendall W.L., Doherty P.F., Hirsch A., Chiarello A.G., Paglia A.P. Use of Atlantic Forest protected areas by free-ranging dogs: estimating abundance and persistence of use. Ecosphere. 2016;7 [Google Scholar]
- Paviolo A., De Angelo C., Ferraz K.M.P.M.B., Morato R.G., Martinez Pardo J., Srbek-Araujo A.C., Beisiegel B.D.M., Lima F., Sana D., Xavier Da Silva M., Velázquez M.C., Cullen L., Crawshaw P., Jorge M.L.S.P., Galetti P.M., Di Bitetti M.S., De Paula R.C., Eizirik E., Aide T.M., Cruz P., Perilli M.L.L., Souza A.S.M.C., Quiroga V., Nakano E., Ramírez Pinto F., Fernández S., Costa S., Moraes E.A., Azevedo F. A biodiversity hotspot losing its top predator: the challenge of jaguar conservation in the Atlantic Forest of South America. Sci. Rep. 2016;6:37147. doi: 10.1038/srep37147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payan E., Oliveira T. Leopardus tigrinus. IUCN red list threat. SPECIES. 2016 T5401263. [Google Scholar]
- Pohl C., Hirsch Hadorn G. Methodological challenges of transdisciplinary research. Natures Sci. Soc. 2008;16:111–121. [Google Scholar]
- Porta A.O. First record of the subfamily Newportiinae (Chilopoda: Scolopendromorpha: Scolopocryptopidae) from Argentina. Rev. LA Soc. Entomol. ARGENTINA. 2016;75:183–185. [Google Scholar]
- Porta A.O., Bertrand M. Nuevas especies de Sellnickiella (Acariformes:Labidosmmatidae) de Sudamérica y Madagascar. In: Rueda-Ramírez D., Moraes G., Carrillo D., Combita-Heredia J., editors. Proceedings Congreso Latinoamericano de Acarología - Speakers Proceedings. Sociedad Latinoamericana de Acarología, Montenegro; Quindío, Colombia: 2016. p. 128. [Google Scholar]
- Prum R.O. Interspecific social dominance mimicry in birds. Zool. J. Linn. Soc. 2014;172:910–941. [Google Scholar]
- Quinete N.S., De Oliveira E.D.S., Fernandes D.R., Avelar A.D.S., Santelli R.E. Assessment of organochlorine pesticide residues in Atlantic Rain Forest fragments, rio de Janeiro, Brazil. Environ. Pollut. 2011;159:3604–3612. doi: 10.1016/j.envpol.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Ratnasingham S., Hebert P. The barcode of life data system. Mol. Ecol. Notes. 2007;7:355–364. doi: 10.1111/j.1471-8286.2007.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratola N., Homem V., Silva J.A., Araújo R., Amigo J.M., Santos L., Alves A. Biomonitoring of pesticides by pine needles - chemical scoring, risk of exposure, levels and trends. Sci. Total Environ. 2014;476:114–124. doi: 10.1016/j.scitotenv.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Rheims C.A. Caayguara, a new genus of huntsman spiders from the Brazilian Atlantic Forest (Araneae: Sparassidae) Zootaxa. 2010;2630:1–29. [Google Scholar]
- Ribeiro M.C., Martensen A.C., Metzger J.P., Tabarelli M., Scarano F., Fortin M.-J. Biodiversity Hotspots. 2011. The Brazilian Atlantic Forest: a shrinking biodiversity hotspot; pp. 405–434. [Google Scholar]
- Ribeiro M.C., Metzger J.P., Martensen A.C., Ponzoni F.J., Hirota M.M. The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009;142:1141–1153. [Google Scholar]
- Rodrigues A.S.L., Andelman S.J., Bakan M.I., Boitani L., Brooks T.M., Cowling R.M., Fishpool L.D.C., Da Fonseca G.A.B., Gaston K.J., Hoffmann M., Long J.S., Marquet P.A., Pilgrim J.D., Pressey R.L., Schipper J., Sechrest W., Stuart S.H., Underhill L.G., Waller R.W., Watts M.E.J., Yan X. Effectiveness of the global protected area network in representing species diversity. Nature. 2004;428:640–643. doi: 10.1038/nature02422. [DOI] [PubMed] [Google Scholar]
- Rossini B.C., Oliveira C.A.M., Gonçalves De Melo F.A., De Araú Jo Bertaco V., Díaz De Astarloa J.M., Rosso J.J., Foresti F., Oliveira C. Highlighting Astyanax species diversity through DNA barcoding. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso J.J., Mabragaña E., Avigliano E., Schenone N., Díaz de Astarloa J.M. Short spatial and temporal scale patterns of fish assemblages in a subtropical rainforest mountain stream. Stud. Neotrop. Fauna Environ. 2013;48:199–209. [Google Scholar]
- Rosso J.J., Mabragaña E., González-Castro M., Delpiani M.S., Avigliano E., Schenone N., Díaz De Astarloa J.M. A new species of the Hoplias malabaricus species complex (Characiformes: Erythrinidae) from the la plata River basin. Cybium. 2016;40 [Google Scholar]
- Rosso J.J., Rueda E.C., Sanchez S., Bruno M.C., Casciotta J., Aguilera G., Almirón A.E., Ruiz Díaz F.J., Cancino D.F., Bugeau B., Mabragaña E., González-Castro M., Delpiani M., Díaz de Astarloa J.M. Basin-scale distribution and haplotype partitioning in different genetic lineages of the Neotropical migratory fish Salminus brasiliensis. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018;28:444–456. [Google Scholar]
- Sabljić A., Güsten H., Verhaar H., Hermens J. QSAR modelling of soil sorption. Improvements and systematics of log KOC vs. log KOW correlations. Chemosphere. 1995;31:4489–4514. [Google Scholar]
- Sassa T., Kihara A. Metabolism of very long-chain fatty acids: genes and pathophysiology. Biomol. Ther. 2014;22:83–92. doi: 10.4062/biomolther.2014.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENASA . 2011. Servicio Nacional de Sanidad y Calidad Agroalimentaria. Endosulfán: nuevas medidas para la importación, elaboración y uso en Argentina (Resolution 511/2011) [Google Scholar]
- Serengil Y., Augustaitis A., Bytnerowicz A., Grulke N., Kozovitz A.R., Matyssek R., Müller-Starck G., Schaub M., Wieser G., Coskun A.A., Paoletti E. Adaptation of forest ecosystems to air pollution and climate change: a global assessment on research priorities. IForest Viterbo. 2011;4:44–48. [Google Scholar]
- Silva W., Metzger J., Simões S., Simonetti C. Relief influence on the spatial distribution of the Atlantic Forest cover on the Ibiúna Plateau. SP. Brazilian J. Biol. 2007;67:403–411. doi: 10.1590/s1519-69842007000300004. [DOI] [PubMed] [Google Scholar]
- Sir E.B., Kuhnert E., Hladkii A.I., Romero A.I. Annulohypoxylon (Hypoxylaceae) species from Argentina. Darwiniana. 2018;6:68–83. [Google Scholar]
- SRH . 2005. Desarrollos de niveles guías nacionales de calidad de agua ambiente correspondientes a clorpirifos. Buenos Aires. [Google Scholar]
- Stork N.E. How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 2018;63:31–45. doi: 10.1146/annurev-ento-020117-043348. [DOI] [PubMed] [Google Scholar]
- Trujillo-Arias N., Calderón L., Santos F.R., Miyaki C.Y., Aleixo A., Witt C.C., Tubaro P.L., Cabanne G.S. Forest corridors between the central Andes and the southern Atlantic Forest enabled dispersal and peripatric diversification without niche divergence in a passerine. Mol. Phylogenetics Evol. 2018:221–232. doi: 10.1016/j.ympev.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Trujillo-Arias N., Dantas G.P.M., Arbeláez-Cortés E., Naoki K., Gómez M.I., Santos F.R., Miyaki C.Y., Aleixo A., Tubaro P.L., Cabanne G.S. The niche and phylogeography of a passerine reveal the history of biological diversification between the Andean and the Atlantic forests. Mol. Phylogenetics Evol. 2017;112:107–121. doi: 10.1016/j.ympev.2017.03.025. [DOI] [PubMed] [Google Scholar]
- USEPA . 2012. United States Environmental Protection Agency, Recreational water quality criteria, OSWER 820-F-12-0582012. Washington, DC, USA. [Google Scholar]
- Van Dyk M.S., Kock J.L.F., Botha A. Hydroxy long-chain fatty acids in fungi. World J. Microbiol. Biotechnol. 1994;5:495–504. doi: 10.1007/BF00367653. [DOI] [PubMed] [Google Scholar]
- Vignale M.V., Grassi E., Robledo G.L. New record for Rickiella edulis (Ascomycota) from Argentina. Bol. Soc. Argent. Bot. 2015;50:237. [Google Scholar]
- Wania F. Assessing the potential of persistent organic chemicals for long-range transport and accumulation in polar regions. Environ. Sci. Technol. 2003;37:1344–1351. [Google Scholar]
- WHO . World Health Organization; Geneva: 1989. Health Guidelines for the Use of Wastewater in Agriculture and Aquaculture. [PubMed] [Google Scholar]
- Williams J.N. Human population and the hotspots revisited: a 2010 assessment. In: Zachos F.E., Habel J.C., editors. Biodiversity Hotspots Distribution and Protection of Conservation Priority Areas. Springer-Verlag Berlin Heidelberg; Berlin: 2011. pp. 61–81. [Google Scholar]
- Wright J.E., Wright A.M. Checklist of the mycobiota of Iguazú national park (Misiones , Argentina) Boletín la Soc. Argentina Botánica. 2005;40:1–22. [Google Scholar]
- WSP World spider catalog. 2019. http://wsc.nmbe.ch online at. accessed on.
- Wu J.X., Wilsey C.B., Taylor L., Schuurman G.W. Projected avifaunal responses to climate change across the U.S. National Park System. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.Q. Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness (Addenda 2013) Zootaxa. 2013 doi: 10.11646/zootaxa.3703.1.1. Mangolia Press, Auckland. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Zhang Y., Wang N., Dong Y., Shou D., Li H. Simultaneous quantification of antioxidant compounds in Phellinus igniarius using ultra performance liquid chromatography-photodiode array detection-electrospray ionization tandem mass spectrometry. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.