ABSTRACT
Pancreatic cancer carries a poor prognosis and given insidious symptoms has often metastasized at the time of presentation. Common sites of metastasis involve liver, lungs, regional lymph nodes, or peritoneum. Colonic metastasis is rare, with only a few previous descriptions in the literature. We report a case of a 91-year-old woman with presumed pancreatic adenocarcinoma based on pathology and imaging, with colonic metastasis presenting as colonic obstruction.
INTRODUCTION
Pancreatic adenocarcinoma has an extremely poor prognosis. The insidious symptoms are often unnoticed at onset until late stage or metastatic changes induce more alarming symptoms. When diagnosed, pancreatic adenocarcinoma has often metastasized, most often to liver, lungs, regional lymph nodes, or peritoneum.1–3 Colonic metastasis is extremely rare, with a few previous descriptions in the literature.1,3–5 We report a case of pancreatic adenocarcinoma with colonic metastasis presenting as colonic obstruction.
CASE REPORT
A 91-year-old woman with a history of hypertension, gastroesophageal reflux disease, and recently diagnosed diabetes mellitus presented with nausea, early satiety, and unintentional 13.6 kg weight loss. She reported a long-standing history of constipation without hematochezia, melena, or change in stool caliber. Initial abdominal radiograph did not reveal distention. Abdominal examination was remarkable for positive bowel sounds and abdominal pain without distention, palpable masses, or jaundice. Laboratory values were not consistent with hepatobiliary obstruction, with alkaline phosphatase of 24 U/L, aspartate aminotransferase of 24 U/L, alanine transaminase of 11 U/L, and total bilirubin of 0.3 mg/dL.
The patient initially received barium swallow to rule out esophageal stricture, and subsequent esophagogastroduodenoscopy revealed gastritis without signs of gastric outlet obstruction due to ulcer or mass. Shortly after, she reported worsening abdominal pain and distention. Repeat abdominal radiograph showed 14-cm dilated right colon (cecum through transverse colon) with retained barium contrast. Computerized tomography (CT) scan was not performed due to concern for artifact and nondiagnostic imaging in setting of previous barium contrast administration.
Flexible sigmoidoscopy was performed after enema preparation, and decompression was attempted; however, severe stenosis of unknown length was found in the sigmoid colon and was unable to be traversed. Patient then underwent a palliative sigmoid colectomy with end colostomy. Follow-up CT with contrast revealed an ill-defined pancreatic head mass measuring 4 × 3.3 cm, encasing celiac artery and proximal branches (Figure 1). Tumor markers were significant for carbohydrate antigen 19-9 of 7,480 U/mL, carcinoembryonic antigen 3.6 ng/mL, and cancer antigen 125 190 U/mL. Pathology confirmed well-differentiated adenocarcinoma of likely metastatic origin. Immunostaining profile showed cytokeratin 7 positive, cytokeratin 20 negative, and caudal-type homeobox transcription factor 2 negative (Figure 2). Given cytokeratin 7 positive and cytokeratin 20 negative, the primary site of adenocarcinoma was presumed to be pancreatic in origin, especially given CT imaging findings.6 The patient desired comfort-focused care and was discharged home with hospice.
Figure 1.
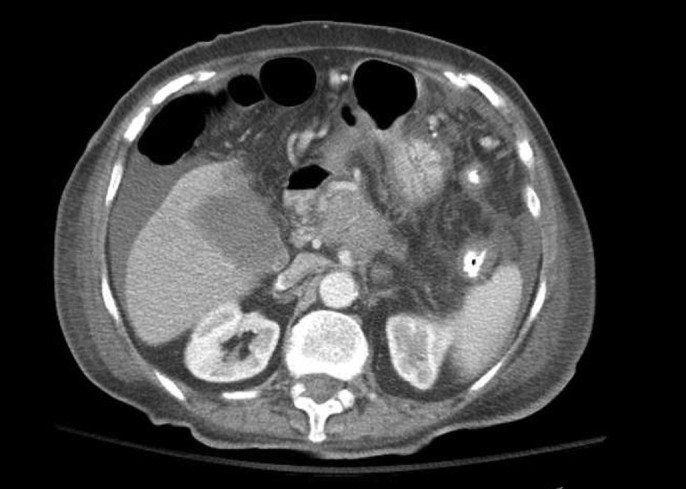
Abdominal/pelvic computed tomography showing a 4.0 × 3.3-cm-diameter mass in the pancreatic head, with encasement of the celiac trunk and proximal branches.
Figure 2.
Resected rectosigmoid lesion with immunostain showing (A) CK7 positive, (B) CK20 negative, and (C) CDX2 negative. CDX2, caudal-type homeobox transcription factor 2; CK7, cytokeratin 7; CK20, cytokeratin 20.
DISCUSSION
Pancreatic cancer continues to carry a very poor prognosis and is the fourth leading cause of cancer deaths in the United States.7,8 The long-term relative survival rate of pancreatic cancer has not substantially changed over the past few decades, and analysis of the most recent decade shows relative survival rate of 28.2% in the first year and 6.9% at 5 years.9 The retroperitoneal location of the pancreas allows for metastatic dissemination to many organs, the most common of which are liver, lungs, regional lymph nodes, and peritoneum.1–3
Colonic metastasis of pancreatic cancer is extremely rare, with a few previously described cases in the literature.1,3–5 Three of the previous cases followed pancreatic lesion resection, and diagnosis was made after pathologic examination.3–5 One previous case, like ours, had an initial presentation with weight loss and imaging suspicious for primary colon malignancy, with the later identification showing pancreatic cancer metastasis.1 Our case is the first case, to our knowledge, of pancreatic adenocarcinoma presenting with sigmoid colon metastasis.
The presentation of pancreatic cancer is often insidious, with nonspecific symptoms such as nausea and anorexia, which delay diagnosis until other more ominous symptoms such as weight loss, abdominal pain, or gastrointestinal symptoms develop.2 Risk factors for pancreatic cancer include tobacco smoking, diabetes mellitus, family history of pancreatitis, metabolic syndrome, and Helicobacter pylori infection.10 Our patient had second-hand tobacco exposure and recently diagnosed with diabetes mellitus. Multiple studies have demonstrated a strong association between pancreatic ductal adenocarcinoma and diabetes mellitus, with the strongest associations within the first few years after diagnosis.11 A 2014 meta-analysis showed a pooled relative risk of 2.08 (95% confidence interval: 1.87–2.32) for pancreatic ductal adenocarcinoma with diabetes than with those without.11 The association between diabetes and pancreatic adenocarcinoma is thought to be mediated by tumor-secreted products and less associated with tumor size or stage.11,12 Current studies attempting to identify serologic markers with increased risk are underway; however, at present, imaging for screening purposes cannot be recommended.12 Her presentation with unintentional 13.6 kg weight loss, early satiety, and colonic obstruction is not a typical presentation of pancreatic adenocarcinoma. Although very rare, the differential for patients presenting with colonic obstruction and concern for colonic mass with a recent diagnosis of diabetes mellitus should include pancreatic adenocarcinoma.
DISCLOSURES
Author contributions: All authors wrote the manuscript. K. George edited the manuscript. K. Patel performed the literature review. R. Kahl is the article guarantor.
Financial disclosures: None to report.
Informed consent was obtained for this case report.
Previous presentation: This case was presented at the World Congress of Gastroenterology at ACG 2017; October 13–18, 2017; Orlando, Florida.
REFERENCES
- 1.Bellows GT, Gage T, Stark M, McCarty C, Haque SC. Metastatic pancreatic carcinoma presenting as colon carcinoma. South Med J. 2009;102:748–50. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra M, Ruggiero M, Zullo A, Serafini S, Grande R, Nardo B. Metastasis of pancreatic adenocarcinoma: A systematic review of literature and new functional concept. Int J Surg. 2015;21(Suppl 1):1–7. [DOI] [PubMed] [Google Scholar]
- 3.Kim W, Lee Y. Metachronus colonic metastasis from pancreatic cancer presenting as mechanical obstruction: A case report. J Clin Imaging. 2015;39(4):699–701. [DOI] [PubMed] [Google Scholar]
- 4.Inada K, Shida D, Noda K, Inoue S, Warabi M, Umekita N. Metachronus colonic metastasis from pancreatic cancer seven years post-pancreatoduodenectomy. World J Gastroenterol. 2013;19:1665–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogu US, Bloch R, Park G. A rare case of metachronus skip metastasis of pancreatic cancer to the colon. Am Surg. 2012;78(7):342–3. [PubMed] [Google Scholar]
- 6.Tot T. Adenocarcinomas metastatic to the liver: The value of cytokeratins 20 and 7 in the search for unknown primary tumors. Cancer. 1999;85(1):171–7. [DOI] [PubMed] [Google Scholar]
- 7.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: A worldwide population-based study (CONCORD). Lancet Oncol. 2008;9:730–56. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 9.Sun H, Ma H, Hong G, Sun H, Wang J. Survival improvement in patients with pancreatic cancer by decade: A period analysis of the SEER database, 1981–2010. Sci Rep. 2014;4(6747):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: A summary review of meta-analytical studies. Int J Epidemiol. 2014;44(1):186–98. [DOI] [PubMed] [Google Scholar]
- 11.Batabyal P, Vander Hoorn S, Christophi C, Nikfarjam M. Association of diabetes mellitus and pancreatic adenocarcinoma: A meta-analysis of 88 studies. Ann Surg Oncol. 2014;21(7), 2453–62. [DOI] [PubMed] [Google Scholar]
- 12.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134(4):981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]



