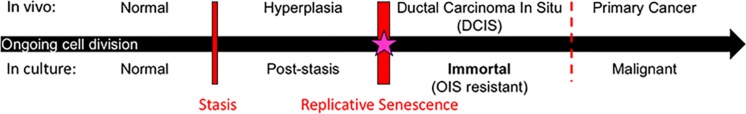
FIGURE 1.
Model of breast cancer progression and barriers for potential chemoprevention targets. Normal cells continue to divide in culture until they approach the stress-associated stasis barrier; cells can bypass stasis by functional inhibition of the retinoblastoma pathway. Post-stasis cells continue to divide until they approach the replicative senescence barrier, which results from ongoing telomere erosion producing telomere dysfunction and genomic instability. Reactivation of telomerase in post-stasis cells can confer immortality. Eroded telomeres, genomic instability, and telomerase reactivation similarly occur at the DCIS stage in vivo. Our research suggests that immortalization coincides with a cancer-unique re-structuring of telomere maintenance mechanisms. Immortalized cells are then resistant to oncogene induced senescence (OIS) and many oncogenes can cause them to become malignant. We propose that the immortalization barrier can be a valuable target for breast cancer prevention (starred).