Table 1. . Phosphonate prodrugs and nomenclature.
| Symmetrical diesters | ||
|---|---|---|
| R’ = | ||
| Alkyl | -(CH2)nCH3 | |
| Aryl | -C6H5 | |
| Benzyl | -CH2C6H5 | |
| Acyloxyalkyl (POM) | -CH2OC(O)C(CH3)3 | |
| Alkoxycarbonyloxyalkyl (POC) | -CH2OC(O)OCH(CH3)2 | |
| S-Acylthioalkyl (SATE) | -CH2CH2SC(O)R | |
| Asymmetrical diesters | ||
| CycloSal |  |
|
| HepDirect | ||
| Monoesters | ||
| Internal monoester | 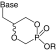 |
|
| Lipid conjugates | 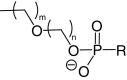 |
|
| Disulfide lipid conjugates | 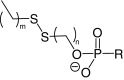 |
|
| Symmetrical bisamidates | ||
| Amino acid esters | 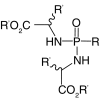 |
|
| Mixed amidate/ester | ||
| ProTides | 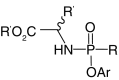 |
|
