Abstract
In this study, tannin-based natural coagulant was used to treat stabilized landfill leachate. Tannin modified with amino group was utilized for the treatment process. Central composite design (CCD) was used to investigate and optimize the effect of tannin dosage and pH on four responses. The treatment efficiency was evaluated based on the removal of four selected (responses) parameters; namely, chemical oxygen demand (COD), color, NH3–N and total suspended solids (TSS). The optimum removal efficiency for COD, TSS, NH3–N and color was obtained using a tannin dosage of 0.73 g at a pH of 6. Moreover, the removal efficiency for selected heavy metals from leachate; namely, iron (Fe2+), zinc (Zn2+), copper (Cu2+), chromium (Cr2+), cadmium (Cd2+), lead (Pb2+), arsenic (As3+), and cobalt (Co2+) was also investigated. The results for removal efficiency for COD, TSS, NH3–N, and color were 53.50%, 60.26%, and 91.39%, respectively. The removal of selected heavy metals from leachate for Fe2+, Zn2+, Cu2+, Cr2+, Cd2+, Pb2+, As3+ and cobalt Co2+ were 89.76%, 94.61%, 94.15%, 89.94%, 17.26%, 93.78%, 86.43% and 84.19%, respectively. The results demonstrate that tannin-based natural coagulant could effectively remove organic compounds and heavy metals from stabilized landfill leachate.
Keywords: landfill, leachate treatment, tannin, coagulation, removal efficiency, heavy metals
1. Introduction
Landfilling is still considered a common and preferable method of disposal for solid municipal waste [1]. However, large quantities of leachate generated from landfills contain high levels of organic and non-organic pollutants, such as heavy metals, dissolved and colloidal solids and various pathogens that can potentially contaminate groundwater and surface water [2,3,4,5,6]. About 133 different toxic chemicals were discovered in 56 conventional municipal waste landfills compared to 72 toxic chemicals in industrial waste landfills [7]. In Malaysia, solid waste generation is expected to reach 30,000 tons per day in 2020, with a daily per capita average of 0.8 kg [8]. The production of solid waste in Malaysia is annually increased between 3% and 4% [9]. This increasing trend is due to rapid economic growth, rapidly changing life styles and rural-urban migration. Approximately 70% of this waste is collected and roughly 95% of collected waste is disposed in landfills [10]. As most of the dumpsites are old, the leachate produced has been stabilized and has low biodegradability [11,12,13]. Therefore, advanced treatment methods are necessary prior to discharge. Several treatment applications have been applied to leachate, such as coagulation and electrocoagulation [14,15], Fenton, photo-Fenton and Electro-Fenton reaction [16,17,18,19], ozonation-based advanced oxidation processes [20,21,22], adsorption and ion exchange [23,24]. In spite of the potential or promise demonstrated by certain treatment processes; questions about treatment cost, sludge production and chemical residues in treated effluent remain unanswered. The use of low cost and natural materials for wastewater treatment has recently gained increasing attention from a number of researchers. In the past two decades, many studies have been conducted to evaluate the effectiveness in substituting chemical coagulants with natural polymers in coagulation processes; polymers have been selected due to a number of advantages: the lower volume of biodegradable sludge generated from their use, their lower toxicity to the environment [25], and their ability to work without requiring pH adjustment for wastewater treatment [26]. Tannins contain positively charged organic compounds with a long polymer chain and can be used as a natural coagulant for industrial wastewater treatment [27]. Tannin is a class of pollutant recognized by its ability to precipitate pollutants of proteins [28].
Application of tannin and its effectiveness for the treatment of low organic water and wastewater has already been demonstrated [29,30,31]. However, few studies have investigated the use of tannin for stabilized leachate treatment. In the present work, the efficacy of tannin-based natural coagulants used for leachate treatment was investigated. Experimental conditions for pH and tannin dosage were optimized, and the removal abilities of the various treatments were determined. Moreover, the efficacy of tannin in removing heavy metals from leachate was also examined.
2. Methodology
2.1. Sampling and Site Characteristics
Leachate samples were collected from Ampar Tenang Closed Landfill Site (ATCL). This landfill site is located in the Sepang district, approximately 4 km to the southeast of Dengkil town in Selangor, Malaysia at latitude 02°48.925′ N and longitude 101°4.933′ E, 40 km southeast of Kuala Lumpur [32]. The landfill site is bound mainly by oil palm plantations, and housing projects have been developed closed to the landfill. The southern area of the site is located approximately 300 m from Labu River. The average precipitation in Dengkil is approximately 2450 mm per year. Annual temperatures consistently range from 24 to 32 °C with a mean temperature of 27 °C [33]. The ATCL is located on the Langat basin alluvial aquifer. Layers from silt and sands represent the shallow confined aquifer; however, the ground surface is more clayey [34], with thickness ranging from 5 to 12 m [35]. There are other layers of clay under the aquifer with thickness ranging between 8 and 15 m that results in the aquifer consisting of lenses on its bottom [36].
The ATCL has a total area of 10 acres. It has been operating for 15 years since 1994. During operation, ATCL received approximately 100 tons of solid waste per day. A total of 500,000 tons of solid waste has been disposed of at the site. The site was fully closed in 2010. ATCL was upgraded from dumping site (Level 0) to sanitary classification (Level 1) before it closed. Leachate samples were collected three times during the August 2017 and January 2018 period. Leachate was manually collected and placed in 500 mL polyethylene containers. The samples were immediately transported to the laboratory and cooled at 4 °C to reduce the biological and chemical reaction. The general characterization for leachate is presented in Table 1.
Table 1.
Descriptive statistics including the mean and standard deviation of leachate quality parameters and heavy metals.
| Parameter | Mean and Standard Deviation | (USEPA [37]; DOE [38]) |
|---|---|---|
| pH | 7.9 ± 0.5 | 6–9 ** |
| EC (µS/cm) | 6565 ± 324 | - |
| TDS (mg/L) | 4671 ± 174 | - |
| TSS (mg/L) | 40 ± 8 | 88 * |
| COD (mg/L) | 893 ± 202 | 400 ** |
| BOD5 (mg/L) | 59 ± 10 | 20 ** |
| NH3–H (mg/L) | 531 ± 22 | 5 ** |
| DO (mg/L) | 5 ± 2 | 10 * |
| Mg (mg/L) | 20 ± 4 | - |
| Ca (mg/L) | 40 ± 3 | - |
| Na (mg/L) | 639 ± 303 | - |
| Fe (mg/L) | 0.8 ± 0.2 | 5 ** |
| Zn (µg/L) | 280 ± 2 | 2000 ** |
| Cu (µg/L) | 42 ± 4 | 20 ** |
| Cr (µg/L) | 45 ± 2 | 10 ** |
| Cd (µg/L) | 0.6 ± 0.1 | 10 ** |
| Pb (µg/L) | 4 ± 1 | 10 ** |
| As (µg/L) | 17 ± 7 | 50 ** |
| Co (µg/L) | 11 ± 8 | 50 ** |
| Mn (µg/L) | 61 ± 49 | 20 ** |
2.2. Tannin Characterization
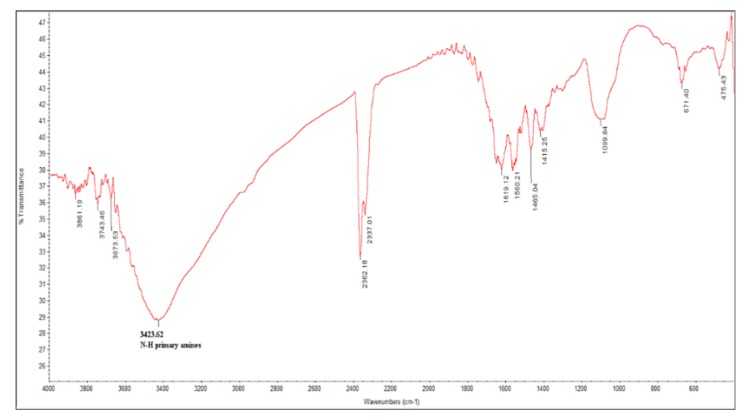
The tannin used in the present study is a commercial variety extracted from the bark of the Black Acacia (Acacia mearnsii) and modified by ammonium [27]. Tannins are hydrolyzable and/or condensed materials [39]. The tannin has a polymeric structure and contains amino groups [28], which are involved in the bridging mechanism used by particles during the coagulation process (Figure 1). The composition and physical properties of naturally extracted tannins are described by Roux et al. [40]. The phenolic building blocks substitution and the aliphatic hydroxyl groups enhanced the reactivity of the natural tannin [40,41,42]. The chemical modification of naturally extracted tannin was reviewed by Arbenz and Avérous [42]. Matamala et al. [43] improved the reactivity of natural tannins extracted from Chilean radiata pine species and from Brazilian black acacia using AISI 1010 (UNS G10100) steels.
Figure 1.
Fourier transformed infrared (FTIR) spectroscopy curve for modified tannin with amino group.
2.3. Experimental Procedure
The experimental procedure was carried out in two stages; the first stage is a preliminary experiment, which was run using one factor a time to identify the region of interest for each influential variable (factor) for pH and tannin dosage and to select the appropriate levels. The selected levels for pH and tannin dosage were used to carry out the second stage using response surface methodology (RSM). A central composite design with two factors pH and tannin dosage and four responses were carried out.
2.3.1. Effect of Tannin Dosage and pH
For the first stage; modified tannin was utilized for the coagulation of stabilized leachate. The different dosages of tannin ranging between 0.25 g and 1.25 g were added separately as a powder form to the 500 mL leachate samples. During this stage of experiment, the initial pH for the leachate sample (8.5) was kept without adjustment, and coagulation was evaluated based on chemical oxygen demand (COD), color, total suspended solids (TSS), and NH3–N removal efficiency. Maintaining the optimum dosage obtained from the previous stage, the influence of pH variation (ranging between three and 12) on the removal of targeted parameters was analyzed. For pH adjustment, 3M of hydrochloric acid solution and 3M of sodium hydroxide solution were used. The pH of all samples was adjusted to the desired value before coagulant addition. Prior to the coagulation process, leachate samples were thoroughly shaken to avoid the possibility of settling solids. The jar test is a method that uses different coagulant dosages to simulate coagulation to determine the minimal dosage required to obtain the highest removal efficiency for targeted parameters. The jar test was first conducted at 250 rpm for 15 min, which was then followed by 60 rpm for 30 min. Then, the liquor was left for 30 min to settle. After settling, the efficacy of various tannin dosages at removing targeted parameters was determined [44].
The removal efficiency for COD was calculated using the following equation, Equation (1):
| Removal (%) = [(Ci − Cf)/Ci] × 100 | (1) |
where Ci and Cf are the initial and final COD concentrations.
2.3.2. Optimization of Treatment Efficiency
A central composite design (CCD) for the tannin-based leachate treatment was created using design expert software (version 6.0.7) to investigate whether COD, color, NH3–N and TSS were influenced by pH values and the various dosages of tannin. The quantities and levels of each variable (factor) were selected based on the preliminary experiments explained in Section 2.3. Thirteen experiments were performed to cover all possible combinations between pH levels and tannin dosages. The levels of the selected factors (pH and tannin) in terms of actual and coded forms are provided in Table 2.
Table 2.
The pH levels and tannin dosages for central composite design (CCD) with axial points.
| Level of Value | Tannin Dosage (g) | pH | ||||
|---|---|---|---|---|---|---|
| Tannin | pH | Coded | Actual | Coded | Actual | Coded |
| 0.4 | 5.17 | −1.414 | 0.5 | −1 | 6 | −1 |
| 0 | 0.75 | 0 | 8 | 0 | ||
| 1.1 | 10.83 | +1.414 | 1 | 1 | 10 | 1 |
The data obtained from various experiments of CCD are usually used to fit a polynomial model and most probably a second-order model (see Equation (2)):
| (2) |
where Y is the response, Xi and Xj are the variables, β is the regression coefficient, k is the number of factors studied and optimized in the experiment, and e is the random error. A p-value less than 0.05 was considered significant.
2.4. Analytical Study
The biochemical oxygen demand (BOD5), chemical oxygen demand (COD), ammoniacal nitrogen (NH3–N), total suspended solid (TSS), electrical conductivity (EC), pH and heavy metals—copper (Cu2+), iron (Fe2+), lead (Pb2+), and zinc (Zn2+)—were determined before and after each run of coagulation. The concentration of BOD5 was determined using Method 5210B. The dissolved oxygen (DO) was measured using a DO meter (model 1000, YSI Inc., Greene County, OH, USA). The COD concentration was determined using the closed reflux colorimetric method (5220B-DR2500 HACH, Loveland, CO, USA). Color concentration was measured using the DR 2800 HACH spectrophotometer at 455 nm wavelength. The pH and EC were measured using a portable digital pH/mV meter (model inoLab pH 720, WTW, Weilheim, Germany). Total suspended solids (TSS) was measured using method 2540C. NH3–N concentration was measured by the phenate method (4500-NH3 F) using a DR2500 spectrophotometer at 640 nm. Heavy metals were analyzed using Atomic Absorption Spectroscopy (Unicam 929 AA Spectrophotometer, UNICO, Franksville, WI, USA). All parameters were determined following standard methods for examination of water and wastewater [45]. Different tannin dosages (0.25, 0.50, 0.75, 1.00 and 1.25 g) were added to raw leachate in the beakers to evaluate its performance in removing targeted parameters.
3. Results and Discussion
3.1. Effect of Tannin
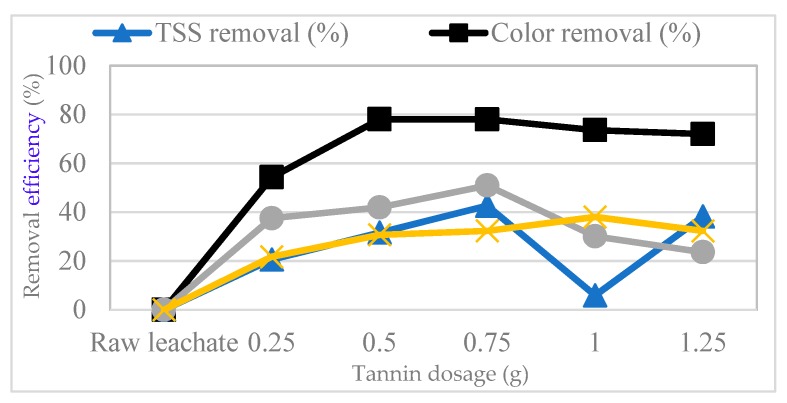
The effects of the various tannin dosages on the leachate samples were investigated. The various tannin dosages ranged between 0.25 g and 1.25 g. Its effects on COD, color, NH3–N and TSS removal were evaluated, as seen in Figure 2. The maximum removal percentage for COD, color and TSS were 54%, 78% and 43%, respectively at 0.75 g of tannin. For ammonia, higher removal was achieved using 1 g of tannin. The removal of targeted parameters was improved when tannin dosage increased between 0.25 g to 0.75 g. The removal of organic pollutants improved due to the ability of natural polyphenols in tannin to adsorb organics and metal ions [46]. The improvement in the removal of organic and ammonia may be due to the effect of electric double layers formed by carboxylic, phenolic and amino groups [47]. The removal efficiency of targeted parameters reduced when higher dosages of tannin (>0.75 g) were applied. The positively charged primary amino groups contained in tannin led to an improved bridging mechanism of the particles and colloids in the leachate and enhanced the flocculation process [27]. Tannin is unhydrolyzed in leachate and has a high molecular weight. The use of higher dosage of tannin leads to fast precipitation of large amount of tannin which may inhibit the flocculation efficiency [48].
Figure 2.
Effect of tannin dosage on chemical oxygen demand (COD), color, total suspended solids (TSS) and NH3–N removal from leachate at a pH of 8.18, 250 rpm for 15 min, 60 rpm for 30 min and settling for 30 min.
3.2. Effect of pH Variation
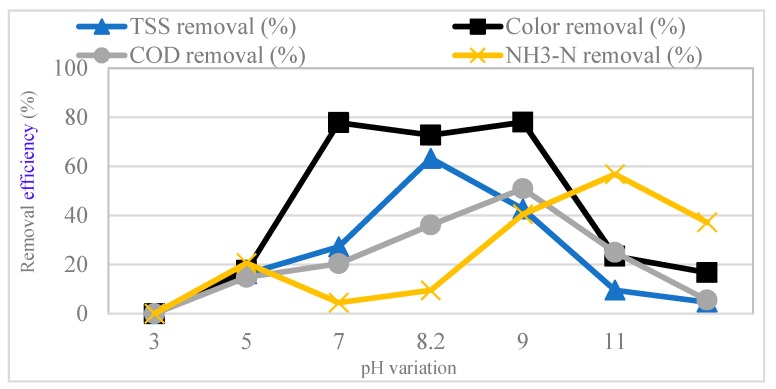
The effect of pH variation in the experiments on leachate coagulation by tannin was investigated by maintaining tannin dosage at 0.5 g. The pH levels used ranged between 3 and 11. Figure 3 shows that the maximum removal for COD (51%) and color (78%) was achieved at pH 9, while the highest removal from TSS (57%) and NH3–N (57%) was achieved at pH 8.18 and 9, respectively. At pH ranging between 7 and 9, the adsorption capability of the particles will be high due to the neutral electric charge [49]. Cations in leachate can improve the coagulation process by neutralizing and destabilizing the negative charges of the residue of the coagulant functional groups by linking with tannin particles [50]. The concentration of monovalent and multivalent cations in leachate, including Mg2+, Ca2+, Na+ and Fe2+, stimulated flocculating activity. These results are analogous to those uncovered by Okaiyeto et al. [51], Wang et al. [52] and Zhang et al. [53], who reported that the multivalent of cations such as Ca2+, Mn2+ and Al3+ increased flocculation activities. Cosa et al. [54] and Nwodo et al. [55] also reported increasing flocculating activity as a result of the influence of Ca2+, Mg2+ and Mn2+.
Figure 3.
Effect of pH variation on COD, Color, TSS and NH3–N removal using a tannin dosage of 0.75 g at 250 rpm for15 min, 60 rpm for 30 min and after 30 min settling.
3.3. Scanning Electron Microscopy (SEM) Imaging
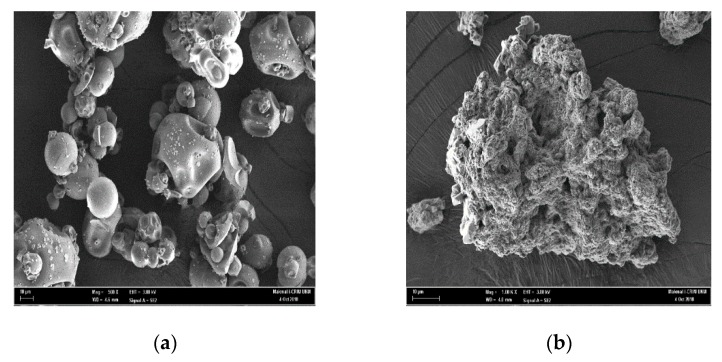
The morphological surface structure of tannin was observed prior to and after the coagulation process. As shown in Figure 4a, tannin has a condensed crystalline brick-shaped structure. The structure served as an attachment site to which suspended particles and cations could bind [56]. Figure 4b illustrates how the coagulant aggregated the particles, which resulted in the formation of larger flocs that were easily settled. Therefore, SEM images of tannin indicated that bridging could be responsible for tannin’s impressive coagulation capabilities [56,57].
Figure 4.
Microscopic image (10 µm) for tannin (a) before (b) after coagulation process observed by scanning electron microscopy.
3.4. Analysis of Variance
The effect of pH and tannin dosages on four responses COD, color, NH3–N, and TSS was investigated using central composite design (CCD). The results of the 13-run (Table 3) obtained from CCD were analyzed using analysis of variance (ANOVA). The normality assumption for the data, which should be checked before starting the analysis, demonstrated that the data for all responses followed normal distribution as presented in Figure 5. The two variables, pH and tannin dosage, showed a significant effect (p-value < 0.05) for the linear or quadratic or both on the selected responses as presented in Table 4 for ANOVA results. Furthermore, the interaction effect between pH and tannin displayed a significant effect on color and TSS removal, which indicates that the two variables pH and tannin dosages do not work independently, while failing to show any significant effect on other removals. This means that the two variables work independently.
Table 3.
Experimental design conditions and actual and predicted removals for the parameters for leachate coagulation with tannin.
| Run No. | Point Type | Factor 1: pH | Factor 2: Tannin (g) | COD Removal (%) | Color Removal (%) | NH3–N Removal (%) | Total Suspended Solids (TSS) Removal (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actual | Predicted | Residual | Actual | Predicted | Residual | Actual | Predicted | Residual | Actual | Predicted | Residual | ||||
| 1 | Axial | 5.17 | 0.75 | 67.00 | 66.90 | 0.10 | 92.00 | 93.43 | −1.43 | 68.90 | 71.20 | −2.29 | 60.26 | 60.64 | −0.38 |
| 2 | Axial | 10.83 | 0.75 | 28.00 | 27.60 | 0.40 | 51.00 | 46.87 | 4.13 | 81.00 | 81.88 | −0.88 | 54.00 | 49.88 | 4.12 |
| 3 | Center | 8.00 | 0.75 | 37.50 | 36.34 | 1.16 | 78.81 | 81.09 | −2.27 | 70.32 | 69.83 | 0.49 | 55.70 | 56.55 | −0.85 |
| 4 | Axial | 8.00 | 0.40 | 34.00 | 37.69 | −3.69 | 59.49 | 59.26 | 0.23 | 52.00 | 54.91 | −2.91 | 45.28 | 44.52 | 0.76 |
| 5 | Center | 8.00 | 0.75 | 36.80 | 36.34 | 0.46 | 83.70 | 81.09 | 2.62 | 71.02 | 69.83 | 1.20 | 55.70 | 56.55 | −0.85 |
| 6 | Fact | 10.00 | 0.50 | 31.00 | 28.57 | 2.43 | 49.00 | 51.52 | −2.52 | 70.00 | 67.98 | 2.02 | 40.00 | 42.67 | −2.67 |
| 7 | Center | 8.00 | 0.75 | 36.17 | 36.34 | −0.16 | 79.00 | 81.09 | −2.09 | 70.79 | 69.83 | 0.96 | 54.40 | 56.55 | −2.15 |
| 8 | Fact | 6.00 | 1.00 | 33.00 | 35.93 | −2.93 | 88.71 | 88.88 | −0.17 | 65.37 | 64.22 | 1.15 | 44.63 | 45.69 | −1.06 |
| 9 | Fact | 10.00 | 1.00 | 11.00 | 14.14 | −3.14 | 44.00 | 48.10 | −4.10 | 73.85 | 73.70 | 0.15 | 42.00 | 46.25 | −4.25 |
| 10 | Axial | 8.00 | 1.10 | 13.00 | 8.81 | 4.19 | 68.00 | 65.53 | 2.47 | 60.00 | 60.27 | −0.27 | 41.00 | 38.02 | 2.98 |
| 11 | Fact | 6.00 | 0.50 | 65.00 | 62.36 | 2.64 | 78.00 | 76.59 | 1.41 | 65.37 | 62.35 | 3.02 | 58.96 | 58.45 | 0.51 |
| 12 | Center | 8.00 | 0.75 | 35.04 | 36.34 | −1.30 | 83.35 | 81.09 | 2.27 | 69.00 | 69.83 | −0.83 | 59.28 | 56.55 | 2.74 |
| 13 | Center | 8.00 | 0.75 | 36.17 | 36.34 | −0.16 | 80.56 | 81.09 | −0.53 | 68.00 | 69.83 | −1.83 | 57.65 | 56.55 | 1.11 |
Figure 5.
Normal probability plots for (I) COD, (II) color, (III) NH3–N and (IV) TSS removals from leachate.
Table 4.
Analysis of variance (ANOVA) for COD, total suspended solids (TSS), color and NH3–N removals.
| Source | Sum of Squares | DF | Mean Square | F Value | Prob > F | |
| COD Removal (%) | Model | 2650.63 | 5 | 530.13 | 103.36 | <0.0001 |
| A | 1414.38 | 1 | 1414.38 | 275.77 | <0.0001 | |
| B | 767.20 | 1 | 767.20 | 149.59 | <0.0001 | |
| A2 | 77.79 | 1 | 77.79 | 15.17 | 0.0059 | |
| B2 | 334.50 | 1 | 334.50 | 65.22 | <0.0001 | |
| AB | 6.81 | 1 | 6.81 | 1.33 | 0.2870 | |
| Residual | 35.90 | 7 | 5.13 | |||
| Lack of Fit | 34.46 | 3 | 11.49 | 31.87 | 0.0030 | |
| Pure Error | 1.44 | 4 | 0.36 | |||
| Cor Total | 2686.53 | 12 | ||||
| Std. Dev: 2.26, R2: 0.9866, Mean: 33.67; Adj R2: 0.9771, C.V: 6.73; Pred R2: 0.9079; Adeq Precision: 34.585 | ||||||
| Color Removal (%) | Source | Sum of Squares | DF | Mean Square | F Value | Prob > F |
| Model | 3003.96 | 5 | 600.79 | 58.23 | <0.0001 | |
| A | 2167.80 | 1 | 2167.80 | 210.10 | <0.0001 | |
| B | 39.36 | 1 | 39.36 | 3.82 | 0.0917 | |
| A2 | 207.87 | 1 | 207.87 | 20.15 | 0.0028 | |
| B2 | 607.43 | 1 | 607.43 | 58.87 | 0.0001 | |
| AB | 61.68 | 1 | 61.68 | 5.98 | 0.0444 | |
| Residual | 72.22 | 7 | 10.32 | |||
| Lack of Fit | 50.44 | 3 | 16.81 | 3.09 | 0.1523 | |
| Pure Error | 21.78 | 4 | 5.44 | |||
| Cor Total | 3076.18 | 12 | ||||
| Std. Dev.: 3.21, R2: 0.9765, Mean: 71.97, Adj R2:0.9598, C.V.:4.46 Pred R2:0.8723, Adeq Precision: 21.336 | ||||||
| Source | Sum of Squares | DF | Mean Square | F Value | Prob > F | |
| NH3–N Removal (%) | Model | 529.27 | 5 | 105.85 | 20.75 | 0.0005 |
| A | 114.12 | 1 | 114.12 | 22.37 | 0.0021 | |
| B | 28.75 | 1 | 28.75 | 5.64 | 0.0493 | |
| A2 | 78.35 | 1 | 78.35 | 15.36 | 0.0058 | |
| B2 | 260.56 | 1 | 260.56 | 51.08 | 0.0002 | |
| AB | 3.71 | 1 | 3.71 | 0.73 | 0.4220 | |
| Residual | 35.71 | 7 | 5.10 | |||
| Lack of Fit | 29.08 | 3 | 9.69 | 5.85 | 0.0604 | |
| Pure Error | 6.62 | 4 | 1.66 | |||
| Cor Total | 564.98 | 12 | ||||
| Std. Dev.: 2.26, R2: 0.9368: Mean: 68.13, Adj R2: 0.8917, C.V.: 3.32: Pred R2: 0.6156, Adeq Precision: 17.580 | ||||||
| TSS Removal (%) | Source | Sum of Squares | DF | Mean Square | F Value | Prob > F |
| Model | 631.55 | 5 | 126.31 | 13.02 | 0.0020 | |
| A | 115.80 | 1 | 115.80 | 11.94 | 0.0106 | |
| B | 42.23 | 1 | 42.23 | 4.35 | 0.0754 | |
| A2 | 2.88 | 1 | 2.88 | 0.30 | 0.6030 | |
| B2 | 405.95 | 1 | 405.95 | 41.84 | 0.0003 | |
| AB | 66.69 | 1 | 66.69 | 6.87 | 0.0343 | |
| Residual | 67.91 | 7 | 9.70 | |||
| Lack of Fit | 53.14 | 3 | 17.71 | 4.80 | 0.0820 | |
| Pure Error | 14.77 | 4 | 3.69 | |||
| Cor Total | 699.46 | 12 | ||||
| Std. Dev.: 3.11, R2: 0.9029, Mean: 51.45, Adj R2: 0.8336, C.V.: 6.05, Pred R2: 0.4267, Adeq Precision:10.690 | ||||||
A: pH B: Tannin dosages.
The effect of pH and tannin was modelled using a second-order model as given in Equations (3)–(6).
| COD removal (%) = +35.88 − 13.30 × A − 9.79 × B + 3.34 × A2 − 6.93 × B2 + 1.30 × A × B | (3) |
| Color removal (%) = +81.09 − 16.46 × A + 2.22 × B − 5.47 × A2 − 9.34 × B2 − 3.93 × A × B | (4) |
| NH3–N removal (%) = +69.83 + 3.78 × A + 1.90 × B + 3.36 × A2 − 6.12 × B2 + 0.96 × A × B | (5) |
| TSS removal (%) = +56.55 3.80 × A − 2.30 × B 0.64 × A2 − 7.64 × B2 + 4.08 × A × B | (6) |
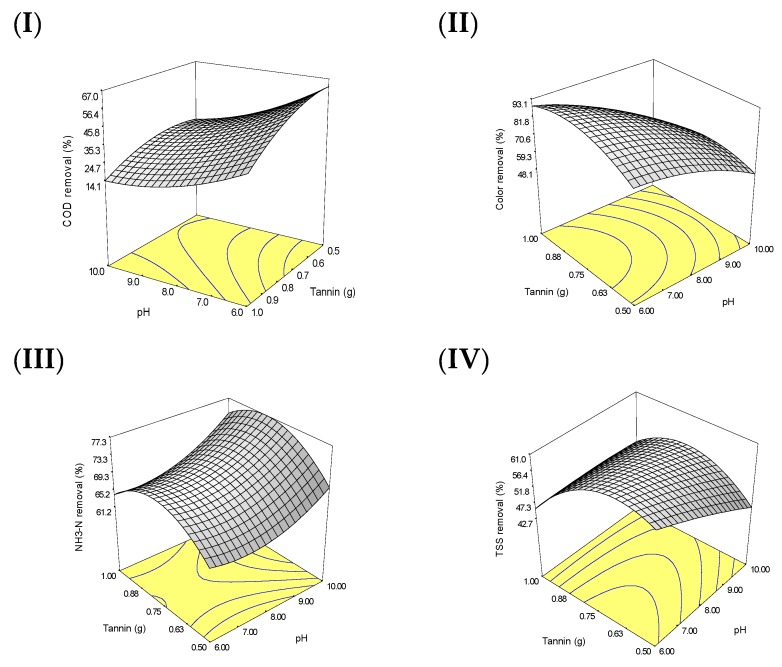
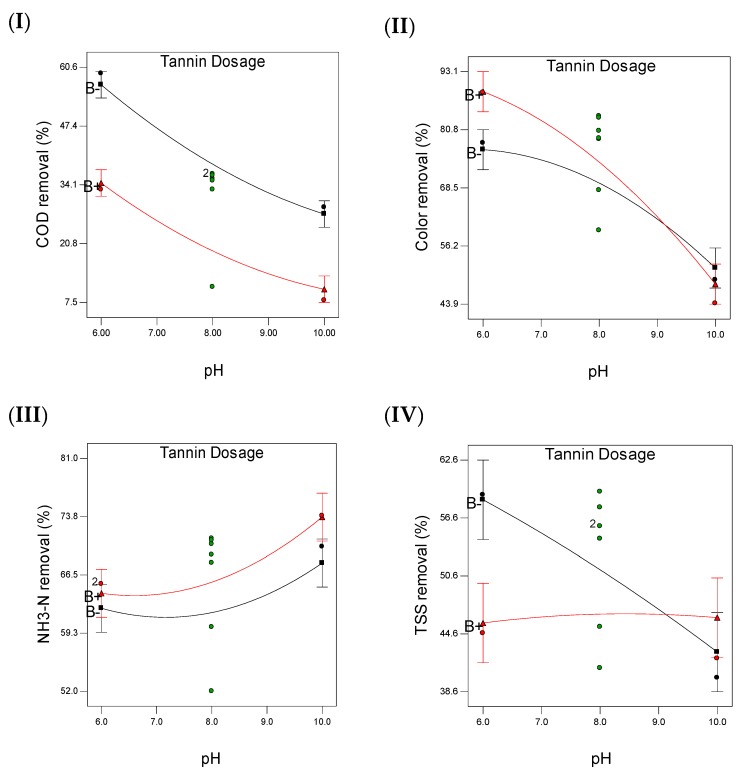
The models in Equations (3)–(6) are good because the coefficient of determination is high and close to 1 (Table 4), which indicates that most of the variance in the data is captured by the model. However, Equation (3) is not as good as other models in explaining the variance in the model since the lack of fit is significant (p < 0.003) which indicates that the model of COD is not as good as other models for color, NH3–N and TSS removals. This means that a higher order model such as third-order polynomial model or more complicated models can be used to enhance the model of COD removal. The effect of pH and tannin on the selected responses is pictorially presented in Figure 6. Figure 6 displays a three-dimensional response surface plot to describe the behavior of each response regarding pH and tannin. Figure 6 demonstrates the maximization of all four responses (COD, color, NH3–N and TSS); the region of maximum effect is well defined with the selected boundaries of pH and tannin. Figure 7 represents the interaction between the two main factors (pH and tannin) and their removal behaviors on the selected parameters.
Figure 6.
Response surface plots for (I) COD, (II) color, (III) NH3–N and (IV) TSS removals from leachate.
Figure 7.
The effect of Interaction between tannin dosage and pH for: (I) COD, (II) color, (III) NH3-N and (IV) TSS removal during coagulation of landfill leachate (B−: represents the low level of tannin dosage, B+: represents the high level of tannin dosage and: represents the design point).
3.5. Optimization of Leachate Treatment Using Tannin
The optimization process was carried out to determine the optimum value of COD, color, NH3–N, and TSS removal efficiency, using the Design Expert 6.0.7 software. According to the software optimization step, the desired goal for each operational condition (tannin dosage and pH) was chosen “within” the range. The responses (COD, color, NH3–N, and TSS) were defined as maximum to achieve the highest performance. The program combines the individual desirability into a single number, and then searches to optimize this function based on the response goal. Accordingly, the optimum working conditions and respective percentage removal efficiencies were established, and the results are presented in Table 5. As shown in Table 5, the removal for COD (53.50%), color (91.4%), NH3–N (69.7%) and TSS (60.7%) are predicted, respectively. The desirability function value was found to be 0.844 for these optimum conditions.
Table 5.
Optimal Response results from model prediction and laboratory.
| pH | Tannin Dosage (g) | TSS Removal (%) | Color Removal (%) | COD Removal (%) | NH3–N Removal (%) | Desirability |
|---|---|---|---|---|---|---|
| 6.00 | 0.73 | 60.3 | 91.3882 | 53.5 | 69.7 | 0.844 |
| Lab experiment | 60.7 | 90.7 | 52.8 | 66.3 | ||
A confirmatory laboratory experiment was run using these optimum conditions, and the results obtained were the following: 60.30% TSS, 90.70% color, 52.80% COD and 66.70% NH3–N removal rates. The residual for COD, color, NH3–N and TSS was reported as 418 mg/L, 279 Pt-Co, 180 mg/L and 17 mg/L, respectively, which are still higher than discharge limits, except for TSS.
3.6. Heavy Metal Removal
The metal complexation of tannin is played an important role for heavy metal deposition from wastewater treatment using natural modified tannin [58]. Slabbert [59] characterized and identified five metal complexes formed by iron (Fe+2), aluminum (Al+3), titanium (Ti), and molybdenum (Mo) ions. The metal complexes increased the charged transfer and enhanced the adsorption and deposition of dissolved heavy metals in water [59,60,61,62,63]. The removal of selected heavy metals from leachate under the obtained optimum experimental conditions (tannin dosage 0.75 g, pH 6, 250 rpm for 15 min followed by 60 rpm for 30 min and settling for 30 min) was investigated, and the results are presented in Table 6. The removal efficiency for the majority of heavy metals ranged between 84% and 94%, while the lowest removal efficiency was reported for Cd2+ at 17.26%, which may be due to the optimal pH value for Cd removal being 5 [64], while the experiment for heavy metals removal was performed at pH 6. Tondi et al. [65] used tannin based rigid foam to enhance the removal of dissolved heavy metals in water. Oo et al. [66] employed mangrove tannins for removing lead (Pb2+) and copper (Cu2+) involving the ion exchange and complexation process by interaction of metals with hydroxyl groups.
Table 6.
Effect of tannin on heavy metal removal (tannin dosage 0.73 g, pH 6, 250 rpm for15 min, 60 rpm for 30 min and 30 min of settling).
| Heavy Metals | Initial Concentration in Leachate | Residual After Tannin Coagulation | Removal (%) |
|---|---|---|---|
| Fe (mg/L) | 0.8 ± 0.2 | 0.1 ± 0.0 | 89 ± 2 |
| Zn (µg/L) | 280 ± 2 | 15 ± 1 | 94 ± 3 |
| Cu (µg/L) | 42 ± 4 | 2.5 ± 0.3 | 94 ± 2 |
| Cr (µg/L) | 45 ± 2 | 5 ± 1 | 90 ± 1 |
| Cd (µg/L) | 0.6 ± 0.1 | 0.5 ± 0.1 | 17 ± 1 |
| Pb (µg/L) | 4 ± 1 | 0.3 ± 0.0 | 94 ± 2 |
| As (µg/L) | 17 ± 7 | 2.4 ± 1 | 86 ± 1 |
| Co (µg/L) | 11 ± 8 | 1.7 ± 0.5 | 84 ± 2 |
4. Conclusions
In this study, the optimization of COD, TSS, color, and NH3–N removal for the coagulation treatment process of stabilized leachate was investigated. In the runs, the highest COD, TSS, color and NH3–N removal rates were achieved at 52.5%, 53.5%, 91.39% and 64%, respectively. Variables such as tannin and pH were modeled with satisfactory degrees of fit. The results suggest that a tannin dosage 0.73 g at a pH of 6 and duration of 45 min can be considered as an efficient pre-treatment process for leachate, and additional post treatment can be considered for further organic and ammonia removals.
Author Contributions
Analysis, T.J.H.B.; Writing-Original Draft Preparation, T.J.H.B.; Supervision, M.M.H., S.S.A.A.; Writing-Reviewing and Editing., M.M.H., S.S.A.A.; Statistical Analysis, A.F.M.A.
Funding
This research was funded by the Universiti Kebangsaan Malaysia (DIP-2017-006) and the Ministry of Education Malaysia (FRGS/1/2018/WAB05/UKM/02/2).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ngoc U.N., Schnitzer H. Sustainable solutions for solid waste management in Southeast Asian countries. Waste Manag. 2009;29:1982–1995. doi: 10.1016/j.wasman.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 2.Aziz H., Rahim N., Ramli S., Alazaiza M., Omar F., Hung Y.T. Potential Use of Dimocarpus longan Seeds as a Flocculant in Landfill Leachate Treatment. Water. 2018;10:1672. doi: 10.3390/w10111672. [DOI] [Google Scholar]
- 3.Kebria D.Y., Ghavami M., Javadi S., Goharimanesh M. Combining an experimental study and ANFIS modeling to predict landfill leachate transport in underlying soil- a case study in north of Iran. Environ. Monit. Assess. 2018;190:26. doi: 10.1007/s10661-017-6374-8. [DOI] [PubMed] [Google Scholar]
- 4.Biswas A.K., Kumar S., Babu S.S., Bhattacharyya J.K., Chakrabarti T. Studies on Environmental Quality in and Around Municipal Solid Waste Dumpsite. Res. Conserv. Recycl. 2010;55:29–134. doi: 10.1016/j.resconrec.2010.08.003. [DOI] [Google Scholar]
- 5.Alkassasbeh J.Y., Heng L., Salmijah S. Toxicity testing and the effect of landfill leachate in Malaysia on behavior of common carp (Cyprinus carpio L., 1758; Pisces, Cyprinidae) Am. J. Environ. Sci. 2009;5:209–217. doi: 10.3844/ajessp.2009.209.217. [DOI] [Google Scholar]
- 6.Tzoupanos N.D., Zouboulis A.I. Water Treatment Technologies for the Removal of High-Toxicity Pollutants. Springer; Dordrecht, The Netherlands: 2009. Characterization and Application of Novel Coagulant Reagent (Polyaluminium Silicate Chloride) for the Post-Treatment of Landfill Leachates; pp. 247–252. [Google Scholar]
- 7.Foo K.Y., Hameed B.H. An overview of landfill leachate treatment via activated carbon adsorption process. J. Hazard. Mater. 2009;171:54–60. doi: 10.1016/j.jhazmat.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Desa A., Kadir N.B., Yusooff F. A Study on The Knowledge, Attitudes, Awareness Status and Behaviour Concerning Solid Waste Management. Procedia-Soc. Behav. Sci. 2011;18:643–648. doi: 10.1016/j.sbspro.2011.05.095. [DOI] [Google Scholar]
- 9.Manaf L.A., Samah M.A.A., Zukki N.I.M. Municipal solid waste management in Malaysia: Practices and challenges. Waste Manag. 2009;29:2902–2906. doi: 10.1016/j.wasman.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Agamuthu P., Fauziah S.H., Kahlil K. Evolution of Solid Waste Management in Malaysia: Impacts and Implications of the Solid Waste Bill, 2007. J. Mater. Cycles Waste Manag. 2009;11:96–103. [Google Scholar]
- 11.Maslahati A., Chelliapan S., Wan Mohtar H., Kamyab H. Prediction and Optimization of the Fenton Process for the Treatment of Landfill Leachate Using an Artificial Neural Network. Water. 2018;10:595. doi: 10.3390/w10050595. [DOI] [Google Scholar]
- 12.Jumaah M.A., Othman M.R., Yusop M.R. Characterization of leachate from Jeram sanitary landfill-Malaysia. Int. J. ChemTech Res. 2016;9:571–574. [Google Scholar]
- 13.Al-Sabahi E., Rahim S.A., Wan Z., Al-Nozaily F., Alshaebi F. The characteristics of leachate and groundwater pollution at municipal solid waste landfill of Ibb City, Yemen. Am. J. Environ. Sci. 2009;5:256–266. [Google Scholar]
- 14.Mussa Z.H., Othman M.R., Abdullah M.P. Electrochemical oxidation of landfill leachate: Investigation of operational parameters and kinetics using graphite-PVC composite electrode as anode. J. Braz. Chem. Soc. 2015;26:939–948. doi: 10.5935/0103-5053.20150055. [DOI] [Google Scholar]
- 15.Labanowski J., Pallier V., Feuillade-Cathalifaud G. Study of organic matter during coagulation and electrocoagulation processes: Application to a stabilized landfill leachate. J. Hazard. Mater. 2010;179:166–172. doi: 10.1016/j.jhazmat.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 16.Aziz H.A., Othman O.M., Abu Amr S.S. The performance of Electro-Fenton oxidation in the removal of coliform bacteria from landfill leachate. Waste Manag. 2013;33:396–400. doi: 10.1016/j.wasman.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Lak M.G., Sabour M.R., Amiri A., Rabbani O. Application of quadratic regression model for Fenton treatment of municipal landfill leachate. Waste Manag. 2012;32:1895–1902. doi: 10.1016/j.wasman.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Mohajeri S., Aziz H.A., Isa M.H., Zahed M.A., Adlan M.N. Statistical optimization of process parameters for landfill leachate treatment using electro-Fenton technique. J. Hazard. Mater. 2010;176:749–758. doi: 10.1016/j.jhazmat.2009.11.099. [DOI] [PubMed] [Google Scholar]
- 19.Mohajeri S., Aziz H.A., Isa M.H., Bashir M.J.K., Mohajeri L., Adlan M.N. Influence of Fenton reagent oxidation on mineralization and decolourization of municipal landfill leachate. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2010;45:692–698. doi: 10.1080/10934521003648883. [DOI] [PubMed] [Google Scholar]
- 20.Abu Amr S.S., Aziz H.A., Adlan M.N. Optimization of stabilized leachate treatment using ozone/persulfate in the advanced oxidation process. Waste Manag. 2013;33:1434–1441. doi: 10.1016/j.wasman.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 21.Rada E., Istrate I., Ragazzi M., Andreottola G., Torretta V. Analysis of electro-oxidation suitability for landfill leachate treatment through an experimental study. Sustainability. 2013;5:3960–3975. doi: 10.3390/su5093960. [DOI] [Google Scholar]
- 22.Goi C.L. A review of marketing mix: 4Ps or More? Int. J. Mark. Stud. 2009;1:2. doi: 10.5539/ijms.v1n1p2. [DOI] [Google Scholar]
- 23.Bashir M.J.K., Aziz H.A., Yusoff M.S. New sequential treatment for mature landfill leachate by cationic/anionic and anionic/cationic processes: Optimization and comparative study. J. Hazard. Mater. 2011;186:92–102. doi: 10.1016/j.jhazmat.2010.10.082. [DOI] [PubMed] [Google Scholar]
- 24.Mojiri A. Review on Membrane Bioreactor, Ion Exchange and adsorption Methods for Landfill Leachate Treatment. Aust. J. Basic Appl. Sci. 2011;5:1365–1370. [Google Scholar]
- 25.Graham N., Gang F., Fowler G., Watts M. Characterisation and coagulation performance of a tannin-based cationic polymer: A preliminary assessment. Colloids Surf. A Physicochem. Eng. Asp. 2008;327:9–16. doi: 10.1016/j.colsurfa.2008.05.045. [DOI] [Google Scholar]
- 26.Vijayaraghavan G., Sivakumar T., Kumar A.V. Application of plant based coagulants for waste water treatment. Int. J. Adv. Eng. Res. Stud. 2011;1:88–92. [Google Scholar]
- 27.Mangrich A.S., Doumer M.E., Mallmann A.S. Química verde no tratamento de águas: Uso de coagulante derivado de tanino de Acacia mearnsii. Revista Virtual de Química. 2014;6:2–15. doi: 10.5935/1984-6835.20140002. [DOI] [Google Scholar]
- 28.Li H., Jiao Y., Xu M., Shi Z., He B. Thermodynamics aspect of tannin sorption on polymeric adsorbents. Polymers. 2004;45:181–188. doi: 10.1016/j.polymer.2003.11.013. [DOI] [Google Scholar]
- 29.Sánchez-Martín J., Beltrán-Heredia J., Solera-Hernández C. Surface water and wastewater treatment using a new tannin-based coagulant. Pilot plant trials. J. Environ. Manag. 2010;91:2051–2058. doi: 10.1016/j.jenvman.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Beltrán-Heredia J., Sánchez-Martín J. Municipal wastewater treatment by modified tannin flocculant agent. Desalination. 2009;249:353–358. doi: 10.1016/j.desal.2009.01.039. [DOI] [Google Scholar]
- 31.Bortolatto J.F., Trevisan T.C., Bernardi P.S.I., Fernandez E., Dovigo L.N., Loguercio A.D., Pretel H. A novel approach for in-office tooth bleaching with 6% H2O2/TiO_N and LED/laser system—A controlled, triple-blinded, randomized clinical trial. Lasers Med. Sci. 2016;31:437–444. doi: 10.1007/s10103-016-1866-2. [DOI] [PubMed] [Google Scholar]
- 32.Mohamed A.F., Yaacob W.W., Taha M.R., Samsudin A.R. Groundwater and soil vulnerability in the Langat Basin Malaysia. Eur. J. Sci. Res. 2009;27:628–635. [Google Scholar]
- 33.Bahaa-eldin E.A.R., Wan Z.W., Rahim S., Ghani R. Geoenvironmental sampling: How good is a good practice. Geol. Soc. Malays. 2003;46:443–446. [Google Scholar]
- 34.Agamuthu P. Heavy Metal Contamination of Soil-Derived Interstitial Water in The Coastal Regions of Selangor Malaysia. Malays. J. Sci. 2001;20:127–134. [Google Scholar]
- 35.Esmail A.S. Master’s Thesis. Universiti Kebangsaan Malaysia; Bangi, Malaysia: 2005. Assessment of Groundwater Pollution in The Vicinity of Ampar Tenang Landfill Site. Unpublished. [Google Scholar]
- 36.Bahaa E.E., Yusoff I., Abdul R.S., Wan Z.W., Ghani M.R. Deterioration of Groundwater Quality in The Vicinity of an Active Open-Tipping Site in West Malaysia. Hydrogeol. J. 2010;18:997–1006. [Google Scholar]
- 37.US Environmental Protection Agency Effluent Limitations Guidelines, Pretreatment Standards and New Source Performance Standards for the Landfills Point Source Category. [(accessed on 10 June 2019)];2000 Available online: https://www.federalregister.gov/documents/2000/01/19/00-1037/effluent-limitations-guidelines-pretreatment-standards-and-new-source-performance-standards-for-the.
- 38.Environmental Quality (Control of Pollution from Solid Waste Transfer Station and Landfill) Regulations Malaysia. FAO; Rome, Italy: 2009. [Google Scholar]
- 39.Oliviero M., Stanzione M., D’Auria M., Sorrentino L., Iannace S., Verdolotti L. Vegetable Tannin as a Sustainable UV Stabilizer for Polyurethane Foams. Polymers. 2019;11:480. doi: 10.3390/polym11030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roux D.G., Ferreira D., Hundt H.K.L., Malan E. Structure, stereochemistry, and reactivity of natural condensed tannins as basis for their extended industrial application. J. Appl. Polym. Sci. 1975;28:335–353. [Google Scholar]
- 41.Tondi G. Tannin-Based Copolymer Resins: Synthesis and Characterization by Solid State 13C NMR and FT-IR Spectroscopy. Polymers. 2017;9:223. doi: 10.3390/polym9060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arbenz A., Avérous A. Chemical Modification of Tannins to Elaborate Aromatic Biobased Macromolecular Architectures. Green Chem. 2015;17:2626–2646. doi: 10.1039/C5GC00282F. [DOI] [Google Scholar]
- 43.Matamala G., Smeltzer W., Droguett G. Comparison of steel anticorrosive protection formulated with natural tannins extracted from acacia and from pine bark. Corros. Sci. 2000;42:1351–1362. doi: 10.1016/S0010-938X(99)00137-7. [DOI] [Google Scholar]
- 44.Ghaffari A., Miller C.C., McMullin B., Ghahary A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide. 2006;14:21–29. doi: 10.1016/j.niox.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 45.American Public Health Association . Standard Methods for the Examination of Water and Wastewater APHA. 21st ed. American Public Health Association (APHA); Washington, DC, USA: 2005. [Google Scholar]
- 46.Palma G., Freer J., Baeza J. Removal of metal ions by modified Pinus radiata bark and tannins from water solutions. Water Res. 2003;37:4974–4980. doi: 10.1016/j.watres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Schofield P., Mbugua D.M., Pell A.N. Review: Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001;91:21–40. doi: 10.1016/S0377-8401(01)00228-0. [DOI] [Google Scholar]
- 48.Kim T.J., Silva J.L., Kim M.K., Jung Y.S. Enhanced antioxidant capacity and antimicrobial activity of tannic acid by thermal processing. Food Chem. 2010;118:740–746. doi: 10.1016/j.foodchem.2009.05.060. [DOI] [Google Scholar]
- 49.Xia T., Kovochich M., Liong M., Mädler L., Gilbert B., Shi H., Nel A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2:2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang B., Su H., Gu X., Huang X., Wang H. Effect of structure and charge of polysaccharide flocculants on their flocculation performance for bentonite suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2013;436:443–449. doi: 10.1016/j.colsurfa.2013.07.017. [DOI] [Google Scholar]
- 51.Okaiyeto K., Nwodo U., Mabinya L., Okoh A. Characterization of a bioflocculant produced by a consortium of Halomonas sp. Okoh and Micrococcus sp. Leo. Int. J. Environ. Res. Public Health. 2013;10:5097–5110. doi: 10.3390/ijerph10105097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L., Feng Z., Wang X., Wang X., Zhang X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2011;26:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- 53.Zhang D., Hou Z., Liu Z., Wang T. Experimental research on Phanerochaete chrysosporium as coal microbial flocculant. Int. J. Min. Sci. Technol. 2013;23:521–524. doi: 10.1016/j.ijmst.2013.07.009. [DOI] [Google Scholar]
- 54.Cosa S., Ugbenyen A.M., Mabinya L.V., Rumbold K., Okoh A.I. Characterization and flocculation efficiency of a bioflocculant produced by a marine Halobacillus. Environ. Technol. 2013;34:2671–2679. doi: 10.1080/09593330.2013.786104. [DOI] [PubMed] [Google Scholar]
- 55.Nwodo U.U., Okoh A.I. Characterization and flocculation properties of biopolymeric flocculant (glycosaminoglycan) produced by Cellulomonas sp. O koh. J. Appl. Microbiol. 2013;114:1325–1337. doi: 10.1111/jam.12095. [DOI] [PubMed] [Google Scholar]
- 56.Salehizadeh H., Shojaosadati S.A. Extracellular biopolymeric flocculants: Recent trends and biotechnological importance. Biotechnol. Adv. 2001;19:371–385. doi: 10.1016/S0734-9750(01)00071-4. [DOI] [PubMed] [Google Scholar]
- 57.He N., Li Y., Chen J., Lun S.Y. Identification of a novel bioflocculant from a newly isolated Corynebacterium glutamicum. Biochem. Eng. J. 2002;11:137–148. doi: 10.1016/S1369-703X(02)00018-9. [DOI] [Google Scholar]
- 58.Lian B., Chen Y., Zhao J., Teng H.H., Zhu L., Yuan S. Microbial flocculation by Bacillus mucilaginosus: Applications and mechanisms. Bioresour. Technol. 2008;99:4825–4831. doi: 10.1016/j.biortech.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 59.Slabbert N. Complexation of Condensed Tannins with Metal Ions. In: Hemingway R.W., Laks P.E., editors. Plant Polyphenols. Basic Life Sciences. Volume 59. Springer; Boston, MA, USA: 1992. pp. 421–436. [Google Scholar]
- 60.Ashraf M.A., Balkhair K.S., Chowdhury A.J.K., Hanafiah M.M. Treatment of Taman Beringin landfill leachate using the column technique. Desalin. Water Treat. 2019;149:370–387. doi: 10.5004/dwt.2019.23839. [DOI] [Google Scholar]
- 61.Basheer A.O., Hanafiah M.M., Alsaadi M.A., Douri Y.A., Aljumaily M.M., Fiyadh S.S. Synthesis and characterization of natural extracted precursor date palm fibre-based activated carbon for aluminum removal by RSM optimization. Processes. 2019;7:249. doi: 10.3390/pr7050249. [DOI] [Google Scholar]
- 62.Hanafiah M.M., Hashim N.A., Ahmed S.T., Ashraf M.A. Removal of chromium from aqueous solutions using a palm kernel shell adsorbent. Desalin. Water Treat. 2018;118:172–180. doi: 10.5004/dwt.2018.22639. [DOI] [Google Scholar]
- 63.Manikam M.K., Halim A.A., Hanafiah M.M., Krishnamoorthy R.R. Removal of ammonia nitrogen, nitrate, phosphorus and COD from sewage wastewater using palm oil boiler ash composite adsorbent. Desalin. Water Treat. 2019;149:23–30. doi: 10.5004/dwt.2019.23842. [DOI] [Google Scholar]
- 64.Rajesh Kumar P.R., Akila Swathanthra P.A., Basava Rao V.V.B., Rao S.R.M. Adsorption of Cadmium and Zinc Ions from Aqueous Solution Using Low Cost Adsorbents. J. Appl. Sci. 2014;14:1372–1378. [Google Scholar]
- 65.Tondi G., Oo C.W., Pizzi A., Trosa A., Thevenon M.F. Metal adsorption of tannin-based rigid foams. Ind. Crops Prod. 2009;29:336–340. doi: 10.1016/j.indcrop.2008.06.006. [DOI] [Google Scholar]
- 66.Oo C.W., Kassim M.J., Pizzi A. Characterization and performance of Rhizophora apiculata mangrove polyflavonoid tannins in the adsorption of copper (II) and lead (II) Ind. Crops Prod. 2009;30:152–161. doi: 10.1016/j.indcrop.2009.03.002. [DOI] [Google Scholar]