Abstract
Starting in 2006, bluetongue virus serotype 8 (BTV8) was responsible for a major epizootic in Western and Northern Europe. The magnitude and spread of the disease were surprisingly high and the control of BTV improved significantly with the marketing of BTV8 inactivated vaccines in 2008. During late summer of 2011, a first cluster of reduced milk yield, fever, and diarrhoea was reported in the Netherlands. Congenital malformations appeared in March 2012 and Schmallenberg virus (SBV) was identified, becoming one of the very few orthobunyaviruses distributed in Europe. At the start of both epizootics, little was known about the pathogenesis and epidemiology of these viruses in the European context and most assumptions were extrapolated based on other related viruses and/or other regions of the World. Standardized and repeatable models potentially mimicking clinical signs observed in the field are required to study the pathogenesis of these infections, and to clarify their ability to cross the placental barrier. This review presents some of the latest experimental designs for infectious disease challenges with BTV or SBV. Infectious doses, routes of infection, inoculum preparation, and origin are discussed. Particular emphasis is given to the placental crossing associated with these two viruses.
Keywords: Bluetongue, Schmallenberg, Culicoides, vector-borne disease, experimental challenge, infection, arboviruses
1. Introduction
Amongst pathogens, RNA viruses were a major source of emerging diseases during the last 30 years [1]. High mutation rate and in case of segmented genome, reassortment are responsible for genetic adaptability and variability of these viruses.
Two pathogens affecting cattle and sheep were responsible for major outbreaks in Mainland Europe in the past 15 years: Bluetongue virus (BTV) and Schmallenberg virus (SBV). These outbreaks were singular in several ways: the diseases were previously either never reported in such northern locations (bluetongue virus) or recently discovered (Schmallenberg virus); their emergence still has unexplained aspects; both viruses displayed the ability to cross the placental barrier. Moreover, these events confirmed that palearctic endemic Culicoides species contribute to the spread of BTV and SBV and to the epizootic aspect of the diseases.
Bluetongue virus causes the eponymous bluetongue disease (BT). BTV belongs to the family Reoviridae, subfamily Sedoreovirinae, and represents the type specie of the Orbivirus genus [2]. The family Reoviridae currently contains fifteen genera of multi-segmented dsRNA viruses, including pathogens of a wide range of vertebrates (including humans), arthropods, plants, and fungi [3]. Unlike the other reoviruses, all orbiviruses are arthropod-borne viruses (arboviruses). This genus currently contains 22 species as well as 10 unclassified “orbiviruses” [4].
Until recent nomenclature changes implemented by the International Committee on Taxonomy of Viruses [5] Schmallenberg virus was part of the Bunyaviridae family, genus Orthobunyavirus, grouped within the serogroup Simbu along with at least 27 other virus species. The members of the Simbu serogroup show cross-reactions to the complement fixation test but are distinguished by seroneutralization [6] and by genetic sequence analysis. Yet still part of the Orthobunyavirus genus, SBV, AKAV and Aino virus (AINOV) are now considered exemplar viruses of the species Sathuperi orthobunyavirus, Akabane orthobunyavirus, and Shuni orthobunyavirus, respectively [7]. These belong to the new order Bunyavirales, family Peribunyaviridae (formerly Bunyaviridae), which comprises the genus Orthobunyavirus and Herbevirus (host range limited to insects).
Despite their belonging to different viral families, BTV and SBV have several features in common. These converging aspects warrant the present work discussing more specifically the elements to consider while designing experimental infections targeting ruminant host species. A particular emphasis will be given to placental crossing and teratogenic potential of these two viruses.
2. Studying the Pathogenesis and Immune Response of BTV and SBV in Natural Ruminant Host Species
Experimental infections of mammalian hosts proved to be a highly valuable tool to study pathogenicity, virulence, pathogenesis, and transplacental infections since the dawn of the study of infectious diseases [8]. Design of in vivo models evolved and were usefully complemented with in vitro and in silico approaches to better comprehend the host-pathogen interactions.
Prior to study the pathogenesis of BTV and SBV in ruminants, including teratogenic potential, experimental models reproducing the disease had to be found.
To date there are no lab-adapted colonies of Palearctic Culicoides. Given the feeding behaviour of Culicoides, investigating the most adapted route of inoculation is of prime importance to ensure standardization and repeatability of challenge experiments. Amongst other important pathogenesis factors to consider when designing experimental infections, the origin of the inoculum and its passage history has to be carefully evaluated. Indeed the number of passages, the cell culture system used to grow the inoculum or by contrast, infectious blood or serum source are central to achieve experimental infection matching virological, clinical, and serological parameters of field infection with wild-type viruses.
2.1. Selection of an Appropriate Inoculum is Crucial to Achieve Adequate Experimental Infection
Adequate inoculum to use in infectious challenges in order to study viral pathogenesis should be:
-
(1)
Safe, meaning it should have been screened for contaminations, adventitial agents or other pathogens [9];
-
(2)
Easily available, practical and standardised; and
-
(3)
Made of a virus displaying similar replication and virulence properties than wild-type.
2.1.1. Infectious Blood versus Cell Passaged Inoculum
A high quality infectious inoculum reproducing the pathogenesis of diseases was essential to investigate vaccine efficacy requirements or certain specific aspects of the pathogenesis of recently discovered viruses [10,11]. Facing an emerging disease with epizootic potential, reproduction of clinical disease under experimental conditions might be more reliable using infectious animal products such as blood or serum. Nevertheless, it appears that in the majority of the most recent experimental infections involving BTV or SBV, cell culture grown inocula were preferred for challenges (Table 1). Reasons to use cell-passaged viruses can be summarized as follow:
-
(1)
Original isolate or any strain of particular interest can be shipped almost anywhere in the world, leading to great improvement of standardization;
-
(2)
Viral amplification by cell-passages allows a high increase in viral titre, subsequently allowing to inoculate lower volumes;
-
(3)
Screening for contamination or other pathogens is easier in cell culture and eliminate some veterinary public health concerns about using ruminant blood to infect other ruminants; and
-
(4)
Virulence in cell culture can be easily standardized.
Table 1.
Inocula characteristics used in the 10 most recent experimental infection studies on BTV and SBV (as searched on PubMed (July 2019) with keywords “experimental infection bluetongue” and “experimental infection Schmallenberg”). Only articles about experimental infection involving at least one ruminant species among cattle, sheep or goats were retained. The number of infected animals only takes into account actually infected ones, excluding control animals.
| Virus | Type of Inoculum | No. and Species of Infected Animals | Cell Type | Number of Passages | Inoculation Route | Volume (mL) | Doses (TCID50/mL) | Reference |
|---|---|---|---|---|---|---|---|---|
| BTV8 | Cell-passaged | 16 sheep | KC | 2 | SC | 1 | 105,75 | Flannery et al., 2019 [12] |
| BTV4 TV16 |
Cell-passaged | 6 sheep | BHK21+KC | not specified | ID | 1 | 106 | Putty et al., 2019 [13] |
| BTV1 BTV2 BTV4 BTV9 BTV16 |
Cell-passaged | 30 cattle | BHK-21 | 2-4 | SC | 2,5-4 | 106 | Martinelle et al., 2018 [14] |
| BTV27 | Cell-passaged blood (goat) | 7 sheep, 13 goats and 4 cattle | BSR; ECE+BSR | 3; 1+3 or 1+2 | SC; IV (blood) | 2, 3 or 4; 1 (blood) | 103–104.67 | Bréard et al., 2018 [10] |
| BTV4 | Cell-passaged | 4 sheep, 3 goats and 3 calves | KC+BHK-21 | 1+1 | SC | 2 – 4 | 106 | Schulz et al., 2018 [15] |
| BTV25 * | Reverse genetic | 10 sheep and 2 goats | / | / | SC+IV | 1 | 105 | van Rijn et al., 2016 [16] |
| BTV8 | Cell-passaged | 8 calves | BHK-21 | 2 | SC+IV | 1–4 | 104–106.15 | Martinelle et al., 2016 [17] |
| BTV8 | Cell-passaged | 10 sheep and 4 cattle | KC | 2 | SC, ID | 1 | 107 | Darpel et al., 2016 [18] |
| BTV8 | Blood | 8 sheep | / | / | ID | 2 | 106.08 | Drolet et al., 2015 [19] |
| BTV8 BTV16 | Cell-passaged | 37 sheep | KC | 3 and 2 | SC | 3 | not possible | Bréard et al., 2015 [20] |
| SBV | Cell-passaged | 13 cattle | BHK-21 | 4 | SC | 10 | 105 | Kęsik-Maliszewska et al., 2019 [21] |
| SBV | Serum (cattle) | 35 cattle | / | / | SC | 2 X 0.5 | not specified | König P et al., 2019 [22] |
| SBV | Cell-passaged/sheep brain homogenate/serum (sheep) | 10 sheep, 9 cattle | C6/36 | 1 | SC | 1 – 3 | 105.15 and 103.15** | Endalew et al., 2019 [23] |
| SBV | Serum (cattle) | 25 goats | / | / | SC | 1 | / | Laloy et al., 2017 [24] |
| SBV | Plasma | 9 sheep | / | / | IV | 20 | not specified | Rodríguez-Prieto et al., 2016 [25] |
| SBV | Serum (cattle) | 5 sheep | / | / | SC | 1 | 103.3 | Poskin et al., 2015 [26] |
| SBV | Serum (cattle) | 17 sheep | / | / | SC | 1 | 103.3 | Martinelle et al., 2015 [27] |
| SBV | Serum (cattle) | 9 sheep | / | / | SC, ID, IN | 1 | 103.3 | Martinelle et al., 2015 [28] |
| SBV | Serum (cattle), blood (sheep) | 6 goats | / | / | SC | 1 | not specified | Laloy et al., 2015 [29] |
| SBV | Serum (cattle) | 12 sheep | / | / | SC | 1 | 103.3, 102.3, 101.3 and 100.3 | Poskin et al., 2014 [30] |
*: actually BTV1 and BTV6 expressing BTV25 proteins. **: converted in TCID50/mL from PFU using the formula PFU (mL)/TCID50 (mL) = 0.7 [31]. ID: intradermic; IN: intranasal; IV: intravenous; SC: subcutaneous.
In several recent studies [14] clinical signs reported in BTV infected animals were of a lesser extent than those reported from the field [14,17,27,28,32,33,34]. As modified live vaccines gain their attenuation through serial cell passages, the first and most obvious hypothesis to explain the mild severity of bluetongue disease in the experiment is the use of culture grown virus. Moreover, passage history of the inocula used involved mostly embryonated chicken eggs (ECE), BHK-21 and VERO cells. It was reported that BTV grown on KC cells (derived from Culicoides sonorensis) could induce a greater clinical signs severity [35] probably because KC cells better mimic natural vector-borne infection compared to virus passaged on other cell lines [36].
Like other families of RNA viruses, Peribunyaviridae-RNA-dependent RNA polymerases and orbiviruses VP1 RNA-dependent RNA polymerase are prone to produce errors during viral genome replication. In general, RNA virus replication is characterized by high mutation rates (10−5–10−3 misincorporations per nucleotide copied), short generation times and high progeny yields [37]. In addition, segmented RNA viruses also generate genomic variations through recombination and reassortment [38]. Therefore, RNA viruses form populations of closely related viral variants that started from a single clone: the quasispecies [39,40].
With arthropod vectors, SBV and BTV typically undergo an alternate two-host ‘life cycle’ and are therefore suggested to be more stable and to evolve slower than vector-independent viruses [41]. Both steps may put selective pressures on these viruses, but it remains unknown whether sequence divergence is related to the mammalian or arthropod portion of life cycle. However, it has been demonstrated that despite the lack of changes in the consensus sequence, the passage of BTV in Culicoides cells induces an increase of the number of low-frequency variants as well as an increase in virulence [42]. This phenomenon has been hypothesized to explain the reduced viraemia and clinical picture seen in the re-emerging BTV8 in 2017 versus the 2007 strain [12].
As a matter of fact, it was also reported that the inoculation of infectious material from field isolates rarely produce a clinical picture as severe as in natural infection [19]. An additional hypothesis would be that Culicoides saliva might act as a catalyser enhancing the ability of BTV to produce severe clinical signs. Indeed Culicoides saliva was demonstrated to contain a trypsin-like protease able to cleave VP2, leading to infectious subviral particles formation with enhanced infectivity [43].
We demonstrated the suitability of BTV8 passaged a few times on cell culture to both reproduce clinical signs and RNA detection in calves [33]. Other authors concluded to the benefits of culture-grown viruses to be used in experimental challenges in ruminants [44], as well. Despite converging results, policy of the OIE remains unchanged regarding recommended vaccine efficacy requirements, i.e., challenging vaccinated and unvaccinated sheep with a virus “passaged only in ruminant animals and with no or limited ECE or cell culture passages” [45].
Results regarding SBV do not show that much consistency. In cattle, Wernike et al. reported a reduced viral replication of cell culture-grown SBV when compared to natural host-passaged inoculum [46]. By contrast, one year later the same team concluded to the suitability of both infectious serum and low passage cell culture material for SBV experimental challenges in sheep [47]. In addition to the passage history, the origin of the isolated virus seems to be of importance as virus originating from the central nervous system failed to reproduce RNAemia in inoculated animals [47]. Successive serial passages in cell-culture usually result in decreased virulence. However, regarding SBV, Varela et al. reported an increased pathogenicity in a SBV strain passaged 32 times in INF-incompetent sheep CPT-Tert cells, associated with a faster spread of the virus in the brain of suckling mice [48]. SBV was demonstrated to grow efficiently in several cell lines including sheep CPT-Tert, bovine BFAE, human 293T, dog MDCK, hamster BHK-21, BSR, KC, and VERO cells [48,49]. Whereas serial passages in CPT-Tert led to the accumulation of a variety of mutations mostly in the M and S segments, the porcine cell line SK-6 proved to be highly susceptible and to allow the genetic stability of SBV throughout successive passages [50]. Therefore, depending on the cell line used to grow SBV, serial passages can lead to attenuation, increased virulence, or efficient propagation with a low frequency of nucleotide exchanges.
2.1.2. A Matter of Doses and Routes
When it comes to arboviruses the choice of the route of inoculation can be driven by two main considerations:
-
(1)
The need for a route that best mimics the behaviour of the vector in field conditions. Usually haematophagous arthropods are either telmophagous or solenophagous; depending on the vector species the route might be intradermal (ID), subcutaneous (SC), or intravenous (IV). In experimental infections the inoculated viral load and volume are usually higher than the ones inoculated through naturally occurring feeding given the size of the arthropods and the size of their mouthparts [51]. Another drawback already mentioned is the lack of vector saliva components, which can modify the structure and infectivity of Reoviridae and Peribunyaviridae viral particles [43,52].
-
(2)
The need for a route that will ensure the virus to reach the blood stream. Quite obviously this is the intravenous route. Since vector saliva components can enhance the infectivity of arboviruses there is a risk that the inoculation of the virus alone or at a distal site from the vector feeding site could result in a failed infection [53]. Therefore, the option to bypass the skin for reaching the bloodstream may be relevant.
Several authors including us [17] used mixed routes to overcome the respective disadvantages of each approach (Table 1; [34,54]). In a study of our group, we compared intranasal, intradermal and subcutaneous routes for experimental infections of ewes with SBV [28]. Intradermal is an interesting yet underused route: indeed most haematophagous arthropods do not pass the skin and their mouthparts only allow them to feed intradermally. Most of the cellular and fluid exchanges between the skin and the blood do occur in the dermis [55]. In addition, there are some evidences suggesting that intradermal inoculation can be more appropriate to reproduces many aspects of natural infection, including clinical disease, viral and immune responses [56]. The intradermic route was demonstrated to better mimic natural early stages of the infection, directly influencing the severity of the disease. BTV-induced immunosuppression is linked to the infection and disruption of follicular dendritic cells, which is mostly possible through intradermic inoculation [57].
However, to perform an actual intradermal inoculation the volume to be injected has to be limited, the dermis being mostly composed of a dense network of collagen fibres. Therefore, it is required to multiply inoculation sites to reach desired total inoculum volume and infectious titre. To realize the inoculation itself, the most practical tools are Dermojet® (Akra Dermojet) or special syringes for intradermal injections (used to perform bovine tuberculosis skin tests for example). These devices allow usually volumes between 0.1 and 0.4 mL, thus the need for multiple injections to reach the common 1–4 mL inoculation volume used in ruminant infectious challenges experiments (Table 1). Moreover, with both systems the inoculum has to be transferred from its original vial to a small tank of the dermojet or to a special cartridge to be used with the intradermal syringe. This extra step increases the number of handlings, which should be limited especially in the case of BSL3 pathogens.
We investigated the intranasal route to test whether or not a potential direct SBV contamination between sheep could be achieved [28]. Regarding BTV several authors reported unexpected and inconclusive direct horizontal transmission with different serotypes (BTV8, BTV1, and BTV26 at least) [58,59,60,61].
Several authors reported a direct link between the inoculated viral doses and the onset of clinical signs and viraemia, i.e., the higher the dose the sooner the clinical signs and viral RNA detection [62,63,64]. In another study, we evaluated four 10-fold dilutions of a SBV infectious serum inoculum in ewes [30]. The undiluted original inoculum had a titre of 2 × 103 TCID50/mL. It appears there is a critical dose to be inoculated for reproducing field-like virological and immunological parameters, and once this threshold is reached, there is no dose-dependent effect anymore. In the successfully infected animals, no statistical differences between the different inoculation doses were found in the duration or quantity of viral RNA circulating in blood, nor in the amount of viral RNA present in virus positive lymphoid organs. Likewise Di Gialleonardo et al. compared three groups of cattle inoculated with 100-fold dilutions of BTV8; no significant differences in viraemia kinetics could be found [65].
Inoculation by the bite of Culicoides was reported to be more efficient than intradermal inoculation, especially by delaying the early immune response of the host despite a generally lower inoculated viral dose when compared to needle inoculation [66]. Several mechanisms were hypothesized to explain this apparently enhanced infectivity in Culicoides transmitted BTV:
-
(1)
The Culicoides saliva contains proteases able to cleave VP2, leading to the formation of infectious subviral particles (ISVP) displaying higher infectivity in KC cells and Culicoides [43];
-
(2)
The ratio of infectious BTV particles versus defective virions produced within Culicoides might be higher when compared with cell culture grown BTV [66]; and
-
(3)
Pharmacological agents contained in Culicoides saliva might affect the host’s immune response by anti-proliferative effects on leucocytes [67] or a reduced INF alpha/beta expression, as demonstrated with vesicular stomatitis virus and mosquito saliva [68].
Nonetheless, the use of Culicoides to perform experimental challenges remains highly limited by practical constraints: to date besides C. nubeculosus, C. riethi, and C. sonorensis no other Culicoides species were successfully establish as lab-adapted colonies [69,70], the alternative being insects caught in the wild. In addition, prior to the infectious challenge on the ruminant host, the infection of Culicoides is particularly tricky given the size of the insect and the exact amount of virus delivered to each ruminant cannot be known.
Altogether, the subcutaneous route seems to represent the best compromise for BTV and SBV. The dose itself has to be sufficient but there is no gain in using massive viral load.
2.1.3. Screening for Concomitant Pathogens
Bluetongue disease history is scarred with incidents of contamination of biological samples. In 1992, modified live vaccines against canine distemper, canine adenovirus type 2, canine parainfluenza, and canine parvovirus, reconstituted with a killed canine coronavirus vaccine, led to abortions in several bitches. A virus could be isolated and was eventually identified as BTV serotype 11 [71,72]. More recently, a case of BTV11 contamination was reported by ANSES (Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail, Maison-Alfort, France), in the context of an experimental infection of goats with BTV8. It appeared to be very closely related to the BTV11 isolated in Belgium [73]. We discussed a BTV15 contamination in a recent study [17]. This particular inoculum has been previously involved in two other experimental infections. Eschbaumer et al. used BTV1 culture supernatant that was then passaged once on VERO cells before being injected in calves and sheep [74]. That inoculum has been subsequently used by Dal Pozzo et al. [34], with the exact same outcome, namely discovery of the BTV15 contamination. BTV inoculums were not only contaminated with BTV heterologous serotypes: Rasmussen et al. reported the use of a BTV2 inoculum contaminated with Border Disease Virus in sheep [75].
So far, literature does not report experimental infections with a SBV inoculum that was contaminated by another virus belonging to the same or a different family. Broadly speaking contamination routes are most likely related to i) laboratory contamination during sample preparation or ii) natural multiple infection of the original donor animal [76]. Given the potential dramatic consequences of such contamination incidents, inocula should be tested for major pathogens affecting the host species used in challenge experiments but also for a set of BTV serotypes considered to be the most at risk. Despite the transient circulation of BTV6 [77], BTV11 [76], and BTV14 [78] of vaccine origin in Europe, the BTV11 contamination here above mentioned happened to be similar to BTV11 reference strain. Hence, the contamination of the inoculum is far from being necessarily related to an ongoing viral circulation even though it might remain silent because of the lack of clinical consequences. Thus, to rule out any potential BTV contamination all known BTV serotypes should be tested for. Such a recommendation would inevitably increase the constraints and costs of quality control of inocula prior to their use in experimental infections. Extensive screening could however be considered on a case-by-case basis.
3. BTV and SBV Display Placental Crossing Abilities and Teratogenic Potential
Vertical transmission from pregnant dams to their offspring is one of the major consequences for both SBV and BTV. Many pathogens are able of crossing the placenta to cause foetal injury. Most maternal virus infections are not transmitted to the foetus. However, certain viruses are able of crossing the placental barrier possibly causing developmental defects (teratogenesis). The teratogenesis is the production of a permanent abnormality in structure or function, restriction of growth, or death of the embryo or foetus [79]. The outcome of in utero infection depends on the susceptibility of the foetus to the infecting virus, which in turn, is a reflection of the gestational age of the foetus at exposure as well as the virulence characteristics of the infecting virus [80]. Nervous tissues are important targets for both BTV and SBV: usually the younger the foetus, the more severe the lesions [81,82].
To colonize the foetus viruses need a way in. Therefore, it is considered that SBV in utero infection can only occur once the first placentomes were established, around day 30 of pregnancy in cattle and slightly earlier in sheep [83,84,85]. At implantation, several changes occur: the papillae in the uterine glands immobilize the conceptus and it starts to elongate (cattle: 15 days post coitum (dpc); sheep: 13-16 dpc). Subsequently the cells of the trophectoderm and the uterine epithelium get interdigitated and binucleate cells start to be seen. Then binucleate cells start to differentiate and to migrate (cattle: 20-22 dpc; sheep: 16–18 dpc). Foetal villi develop in the caruncular areas starting at 24–26 dpc in small ruminants and 28–30 dpc in cattle, thus defining the end of the implantation and the start of the placental development [86]. Table 2 summarizes some of the essential events in the course of the prenatal development in cattle and sheep.
Table 2.
Key events in sheep and cattle embryos/foetuses with particular emphasis on nervous and immune systems. Compiled from [87,88,89,90,91,92,93].
| Event | Timing in Cow (dpc) | Timing in Sheep (dpc) |
|---|---|---|
| Blastocyst hatching from zona pellucida | 9 | 9 |
| Elongation of the blastocyst, establishment of the primitive streak, emergence of the notochord | 17–18 | 13–14 |
| Appearance of neural folds, closure of the neural groove | 17–19 | 15–16 |
| Implantation begins | 16–19 | 15–18 |
| Neurula | 20–21 | 17 |
| Neural tube complete; optic and otic vesicles present | 21–23 | 19–20 |
| Placentation begins | 22–23 | 17–22 |
| Three brain vesicles visible | 24–25 | 17 |
| Placentoma are detectable | 32–36 | 21 |
| Lymphoid development of the thymus | 42 | 36 |
| Spleen development | 55 | 43–44 |
| Peripheral lymph nodes | 60 | 45 |
| IgM containing cells | 59 | 65 |
| Myelin sheath acquisition (starting) | 60 | 54–63 |
| IgG containing cells | 145 | 87 |
With dpc, the days post coitum; IgM, immunoglobin M; IgG, immunoglobin G
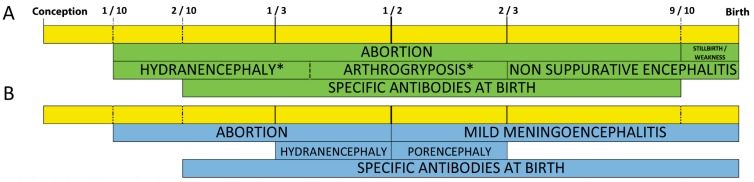
In a recent study, we decided to infect with BTV8 vaccinated and non-vaccinated pregnant heifers at 120 days of pregnancy (BTV pregnant heifers study [32]). We also challenged pregnant ewes with SBV at 45 and 60 days of pregnancy (SBV pregnant ewes study [27]). Thus, for both viruses the experimental infection took place within the critical timeframe, between 30 and 150 days for cattle and between 30 and 70 days of pregnancy for sheep (Figure 1, [85]). Moreover, in experimental conditions the most frequent BTV transplacental infection was reported to occur at mid-term gestation, around 70 days of pregnancy in sheep [58,59].
Figure 1.
Schematic suggested timeframe for SBV (A) and BTV (B) in utero infection causing defects in cattle and small ruminants’ offspring [27,32,81,85,104,127,128]. In utero potential BTV (blue shades) and SBV induced defects (green shades) following infection of the pregnant dams along the whole gestation time for cattle and small ruminants. Time is expressed as a fraction of the total gestational time. A more detailed timeline is available as supplementary file.
The prenatal period can be divided into four main periods: i) fertilization; ii) blastogenesis; iii) embryogenesis; and iv) foetogenesis [94]. The embryo develops tissues and organ structures from the three original germ layers (ecto-, meso-, and endoderm). Once the organs are differentiated the embryo becomes a foetus [95]. The foetal phase is characterized by a fast growth of the conceptus. In cattle and sheep the foetogenesis starts around 45 and 38 dpc, respectively [90]. Thus, the critical timeframe for BTV and SBV infection overlaps the end of the embryo stage and the beginning of the foetal stage. Moreover, although in ruminants gamma globulins are unable to go through the placental barrier from the mother to the foetus it is admitted that cow and sheep foetuses become sequentially and increasingly immunocompetent to a larger variety of antigens throughout the pregnancy [96,97]. The critical timeframe for BTV and SBV infection also spans over the course of several important events during the immune system development (Table 2). Although the sequence of antigens to be successively and progressively recognized by the foetal ruminant through pregnancy seems to be quite conserved between individuals, these antigens can be recognized starting with a difference of a few days between individuals [98]. This individual variability could explain the findings by De Clercq et al. (2008), who reported all possible combinations of serological status/RTqPCR results in dam/calf pairs in a context of high BTV8 suspicion along with results which were interpreted as apparent immunotolerance [99]. Likewise, malformed calves and lambs were reported to be SBV viropositive or vironegative with or without SBV antibodies, suggesting the possibility of an in utero clearance of the virus. Moreover, most of the malformed calves that were negative in both SBV antibodies and RTqPCR were born from seropositive mothers [100].
In our studies, none of these evocative lesions was reported either in the BTV pregnant heifers study or in the SBV pregnant ewes study [27,32].
Following the infection of pregnant dams we reported the reddening of the muzzle and haemorrhages in the wall of the pulmonary artery in calves born from non-vaccinated mothers. Haemorrhages of the pulmonary artery is a BTV typical yet not pathognomonic lesion [101]. These findings were associated with the absence of any anti-BTV antibodies prior to the colostrum intake [32]. Melzi et al. reported the early infection and destruction of follicular dendritic cells following BTV infection. Consequently, antibody production is notably impaired and could be an element explaining the lack of BTV antibody detection in those calves [57]. This result provides an interesting perspective to the many petechial haemorrhages we observed on lymph nodes in several of our own experimental infections [14,17,32,33].
Following the infection of pregnant ewes with SBV, out of the 22 born-alive lambs none had any anti-SBV neutralizing antibodies prior to colostrum intake [27].
In both these experiments, timing of inoculation was optimal to achieve transplacental infection of the foetus with regard to data available from the literature yet no malformations could be seen. No antibodies against the virus used to infect the mothers could be detected as well. These striking results might even question the success of the infection, notwithstanding the positive RNA detection in the mothers. In our BTV pregnant heifers study, the report of similar lesions and serology results in another experiment on goats [102], and in our SBV pregnant ewes study the detection of SBV nucleic acids in organs of several lambs and many extraembryonic structures provided support to an actual transplacental infection. In addition, in another study [103] we managed to isolate SBV from foetal envelopes in the animals from the SBV pregnant ewes study at birth, thus 90 and 105 days post infection. The very low ratio of precolostral seroconversion in immunocompetent foetuses was also reported following the infection of pregnant cattle with SBV [104].
Transplacental transmission of BTV8 based on field data was reported to range from 16% [105,106] to 35% [107,108]. In experimental infections, passage of BTV8 from the mother to the foetus could be demonstrated in 43% of infected ewes whereas BTV1 could be detected in up to 67% of the foetus [59]. The highest susceptibility could be observed around 35-42 days of pregnancy in sheep [109] and infections after day 75 were reported to result in much lighter consequences [110]. Placental crossing, depending on the gestational stage, the BTV serotype and the inoculated dose, was reported to cause congenital defect in up to 40% of the offspring of infected ewes [111]. Other authors observed a BTV8 vertical transmission rate of 33% in goats infected at 61 days of pregnancy [112].
During the BTV epizootic of 2007–2008, Darpel et al., estimated transplacental infection rate of 33%, which is consistent with the latter result [107].
The lesions potentially presented by the calves affected in utero by SBV could be distinguished according to two entities: a hydrocephaly/hydranencephaly syndrome and a torticollis/arthrogryposis syndrome. By analogy with Akabane virus the infection during the first 6 months seems to be critical: an infection of the foetus between 76 and 104 days usually gives rise to hydranencephaly/porencephaly type lesions, and from 103 to 174 it is predominantly arthrogryposis [113]. The latest lesions have been observed for infection at 249 days of gestation and it appears that foetuses less than two months old (after conception) could be protected from in utero infection [113]. In contrast to SBV torticollis was hardly seen after a BTV8 in utero infection during the BTV epidemic in 2006–2009 but was more dominated by hydranencephaly in sheep [114].
Also in contrast to SBV, BTV in utero infection is in the vast majority of cases associated to BTV lab adapted strains (i.e., passaged on cell culture like modified live vaccine strains) or more recently with the European BTV8 wild type virus. By the end of the 20th century, at least five BTV serotypes of modified live vaccine origin (BTV4, BTV10, BTV11, BTV13, BTV17) were reported to be able to cross the placental barrier and possibly causing teratogenic effects [115,116,117,118]. In utero infection caused by wild-type strains was considered uncommon [119] yet documented [120].
SBV vertical transmission seems to be lower when compared to BTV, especially in cattle [121]. The rate of malformations caused by SBV was reported to be about 0.5% in cattle [122] although the rate of intrauterine infection—based on serological results of the calves prior to colostrum intake—was reported to be up to 28% [83]. Other authors documented field data about congenital malformations affecting 3% of the calves but 8-10% of the lambs in farms at the beginning of the SBV epizootic [123,124]. In Belgium based on a survey targeting farmers we also found an estimated 10% of malformed sheep in SBV positive flocks [125].
Table 3 summarizes the most common in utero malformations and nervous lesions induced by some of the most common viruses inducing BTV and SBV-like lesions in ruminants.
Table 3.
Summary of some of the most common central nervous and musculoskeletal lesions following in utero infection with bovine virus diarrhoea virus (BVDV), SBV, BTV, Akabane virus (AKAV), or Aino virus (AV). Adapted from [126].
| Lesion | Definition | BVDV | SBV | BTV | AKAV/AV |
|---|---|---|---|---|---|
| Hydranencephaly | Extensive loss of cerebral tissue with replacement by clear fluid | + | + | + | + |
| Porencephaly | Cystic fluid filled cavities in the brain tissue | + | + | + | + |
| Hydrocephalus | Dilation of the lateral ventricles by cerebrospinal fluid | + | + | + | - |
| Microencephaly | Reduced size of the cerebrum | + | + | + | + |
| Cerebellar hypoplasia | Reduced size of the cerebellum | + | + | + | |
| Kyphosis | Dorsal vertebral column curvature | - | + | - | - |
| Lordosis | Ventral vertebral column curvature | - | + | - | - |
| Scoliosis | Lateral vertebral column curvature | - | + | - | - |
| Torticollis | Twisted cervical vertebral column curvature | - | + | - | - |
| Arthrogryposis | Joint contraction of the limbs | - | + | +/- | + |
Embryonic losses represent a key factor affecting ruminant production systems. In cattle, as well as in sheep, most of the spontaneous embryo mortalities occur in the early embryonic life, namely before 16–18 dpc [129,130]. In cattle early embryonic losses under normal conditions were reported to range from 20 to 44% whereas in sheep in ranges from 12 to 30%, with a clear increase of embryo deaths with the ovulation rate [129,131]. The impact of both BTV and SBV on reproductive parameters other than teratogenesis is well documented. BTV8 was reported to increase the 56-days-return to service rate and the number of AI (Artificial Insemination) required to achieve pregnancy [132,133]. During the SBV epizootic the number of AI to get cattle pregnant was slightly yet significantly increased regardless of whether or not they were part of a herd reporting malformations indicative of an actual infection [134].
Although SBV and especially BTV had a tremendous economic impact on livestock industry, it is worthwhile highlighting the relative poor efficiency of the placental crossing and more specifically the overall low rate of congenital deformities induced by those viruses. However, congenital malformations underestimate the actual rates of BTV and SBV transplacental infections [128].
4. Conclusion and Future Prospects
The number of ruminants used in experimental infections is chosen based on welfare and statistical concerns but also quite unfortunately on economic and practical grounds [8]. We performed our experimental infections with BTV in the BSL3 facilities of Sciensano (Ukkel, Belgium) and with SBV in BSL2+/BSL3 facilities depending the phase of the experiment. Indeed, the Belgian Service of Biosafety and Biotechnology as well as the Belgian law classify BTV as a class 3 pathogen whereas there is no recommendation for SBV. Our biosafety measures for SBV were based on the analogy with AKAV, also classified as a class 3 pathogen [135]. Domestic ruminants being herd animals, need to be housed in groups or at least not individually. Euthanasia methods have to be the most humane as possible and clear end points have to be defined. Given the scarcity of clinical signs caused by BTV and SBV in the field and the individual variations in the response to the infection the number of animals to be included has to be chosen very carefully to comply with the Reduction objective (Three Rs concept) but has to be sufficient to limit the risk of not being able to provide useful data in the context of the ongoing scientific investigation. This is particularly difficult for experimental infection of pregnant ruminants with low malformation rates following transplacental transmission.
The most objective parameter to assess a vaccine efficacy against a virus and especially a RNA vector-borne virus is the evaluation of the viral RNA detection by RTqPCR in the host target [44]. BTV and SBV virulence was demonstrated to vary depending on the ruminant host whether it is cattle, sheep or goat. In addition, pregnancy length differs between cattle and small ruminants while the placentation and the development of the foetal envelopes present slight differences [136]. Consequently, to study any of the specific aspects related to a ruminant species there are no other animal models or any alternative able to mimic the natural situation in a proper way [8].
The results presented in our latest studies provide new insights in viruses that spread through Europe causing severe losses in livestock industry. In addition, these aspects open new perspectives to expand the knowledge on emerging vector-borne viruses targeting ruminants. More specifically, according to our experiments, the subcutaneous route with an inoculum passaged a limited number of times on cell culture seems to represent the best compromise between a high probability to reproduce an infection similar to what happens in the field and logistics concerning the preparation/storage/management of the inoculum. To prevent the loss of viral variability and limit the risks of attenuation, isolation of BTV could be done on KC cells [35], whereas SBV could benefit from an isolation on the highly susceptible SK-6 cell line [50]. Screening for concomitant pathogens should be considered on a case by case basis, if required. The dose should be chosen based on literature data yet no advantage is provided by inoculating a massive viral load.
Since some data from other authors suggest a better reproduction of the diseases with intradermal inoculation, it could be further investigated, especially if more user-friendly devices would be available. A major breakthrough would be the successful adaptation of a colony of Palearctic BTV and SBV vector Culicoides species (C. obsoletus/scoticus, pulicaris) to laboratory conditions and subsequent use in infectious challenges. Vector-borne transmission of BTV implies the puncture of the skin at some point. There is growing evidence that under certain circumstances additional routes of transmission can be observed: a goat was reported to be infected by BTV2 without direct contact [75]. The recently discovered BTV26 also displayed the ability to infect goats through direct contact [61]. In other experimental infections control ewes were found positive with BTV1 and BTV8 [58,59]. The study of the virus factors affecting this modified/underreported transmission feature should allow a better understanding of the epidemiology of the disease.
In conclusion, targeting ruminant host species in experimental infections especially with BSL3 Culicoides borne pathogens is very expensive, time consuming, subject to stringent animal welfare constraints and critical sample size analysis to meet optimal statistical requirements. However, ruminant model remains unavoidable to assess the disease impact and to study the pathogenesis of emerging vector-borne viruses.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/11/8/753/s1, Figure S1: Schematic suggested timeframe for SBV (A) and BTV (B) in utero infection causing defects in cattle and small ruminants’ offspring [27,32,81,85,104,127,128].
Author Contributions
L.M., C.S. and F.D.P. conceived the review. L.M. wrote the review paper. C.S., F.D.P., K.D.C. and E.T. reviewed the article. Graff Sophie and K.D.C. improved the English. All authors read and approved the final manuscript.
Funding
Fundings by the Federal Public Service Health, Food Chain Safety and Environment (contract RF 6190, contract RT 10/10 BLUETONGUE, contract RT 12/6270, and contract RF 12/6290), Brussels, Belgium, by the “Fonds Spéciaux pour la Recherche – Crédits de démarrage” (contract D-08/26), University of Liège, Belgium, by the “Fonds Spéciaux pour la Recherche – Gros équipements” (contract GE-08/02), University of Liège, Belgium, by the FRS-FNRS (FRFC Grant 2.4611.11), and by the European Union as outlined in Council Decision 2012/349/EU concerning a financial contribution by the Union for studies on Schmallenberg virus. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Carrasco-Hernandez R., Jácome R., Vidal Y.L., De León S.P. Are RNA Viruses Candidate Agents for the Next Global Pandemic? A Review. ILAR J. 2017;58:343–358. doi: 10.1093/ilar/ilx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mertens P.P., Maan S., Samuel A., Attoui H. Orbivirus, Reoviridae. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus Taxonomy, VIIIth Report of the ICTV. Elsevier/Academic Press; Oxford, UK: 2005. pp. 466–483. [Google Scholar]
- 3.Mertens P.P., Diprose J., Maan S., Singh K.P., Attoui H., Samuel A.R. Bluetongue virus replication, molecular and structural biology. Vet. Ital. 2004;40:426–437. [PubMed] [Google Scholar]
- 4.Attoui H., Maan S., Anthony S.J., Mertens P.P.C. Bluetongue. Volume 1. Elsevier/Academic Press; London, UK: 2009. Bluetongue virus, other orbiviruses and other reoviruses: Their relationships and taxonomy; pp. 23–52. [Google Scholar]
- 5.Adams M.J., Lefkowitz E.J., King A.M.Q., Harrach B., Harrison R.L., Knowles N.J., Kropinski A.M., Krupovic M., Kuhn J.H., Mushegian A.R., et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017) Arch. Virol. 2017;162:2505–2538. doi: 10.1007/s00705-017-3358-5. [DOI] [PubMed] [Google Scholar]
- 6.Kinney R.M., Calisher C.H. Antigenic relationships among Simbu serogroup (Bunyaviridae) viruses. Am. J. Trop. Med. Hyg. 1981;30:1307–1318. doi: 10.4269/ajtmh.1981.30.1307. [DOI] [PubMed] [Google Scholar]
- 7.De Regge N. Akabane, Aino and Schmallenberg virus-where do we stand and what do we know about the role of domestic ruminant hosts and Culicoides vectors in virus transmission and overwintering? Curr. Opin. Virol. 2017;27:15–30. doi: 10.1016/j.coviro.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Coetzee P., van Vuuren M., Venter E.H., Stokstad M. A review of experimental infections with bluetongue virus in the mammalian host. Virus Res. 2014;182:21–34. doi: 10.1016/j.virusres.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speder B. In: Regulatory Requirements for Viral-Challenge Studies: Influenza Case Study. SGS Life Science Services, editor. SGS Life Science Services; London, UK: 2014. p. 5. [Google Scholar]
- 10.Bréard E., Schulz C., Sailleau C., Bernelin-Cottet C., Viarouge C., Vitour D., Guillaume B., Caignard G., Gorlier A., Attoui H., et al. Bluetongue virus serotype 27: Experimental infection of goats, sheep and cattle with three BTV-27 variants reveal atypical characteristics and likely direct contact transmission BTV-27 between goats. Transbound. Emerg. Dis. 2018;65:e251–e263. doi: 10.1111/tbed.12780. [DOI] [PubMed] [Google Scholar]
- 11.MacLachlan N.J., Nunamaker R.A., Katz J.B., Sawyer M.M., Akita G.Y., Osburn B.I., Tabachnick W.J. Detection of bluetongue virus in the blood of inoculated calves: Comparison of virus isolation, PCR assay, and in vitro feeding of Culicoides variipennis. Arch. Virol. 1994;136:1–8. doi: 10.1007/BF01538812. [DOI] [PubMed] [Google Scholar]
- 12.Flannery J., Sanz-Bernardo B., Ashby M., Brown H., Carpenter S., Cooke L., Corla A., Frost L., Gubbins S., Hicks H., et al. Evidence of reduced viremia, pathogenicity and vector competence in a re-emerging European strain of bluetongue virus serotype 8 in sheep. Transbound. Emerg. Dis. 2019;66:1177–1185. doi: 10.1111/tbed.13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putty K., Shaik A.M., Peera S.J., Reddy Y.N., Rao P.P., Patil S.R., Reddy M.S., Susmitha B., Jyothi J.S. Infection kinetics and antibody responses in Deccani sheep during experimental infection and superinfection with bluetongue virus serotypes 4 and 16. Vet. World. 2019;12:41–47. doi: 10.14202/vetworld.2019.41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinelle L., Dal Pozzo F., Thys C., De Leeuw I., Van Campe W., De Clercq K., Thiry E., Saegerman C. Assessment of cross-protection induced by a bluetongue virus (BTV) serotype 8 vaccine towards other BTV serotypes in experimental conditions. Vet. Res. 2018;49:63. doi: 10.1186/s13567-018-0556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz C., Sailleau C., Bréard E., Flannery J., Viarouge C., Zientara S., Beer M., Batten C., Hoffmann B. Experimental infection of sheep, goats and cattle with a bluetongue virus serotype 4 field strain from Bulgaria, 2014. Transbound Emerg Dis. 2018;65:e243–e250. doi: 10.1111/tbed.12746. [DOI] [PubMed] [Google Scholar]
- 16.van Rijn P.A., van de Water S.G.P., Maris-Veldhuis M.A., van Gennip R.G.P. Experimental infection of small ruminants with bluetongue virus expressing Toggenburg Orbivirus proteins. Vet Microbiol. 2016;192:145–151. doi: 10.1016/j.vetmic.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Martinelle L., Dal Pozzo F., Sarradin P., Van Campe W., De Leeuw I., De Clercq K., Thys C., Thiry E., Saegerman C. Experimental bluetongue virus superinfection in calves previously immunized with bluetongue virus serotype 8. Vet. Res. 2016;47:73. doi: 10.1186/s13567-016-0357-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darpel K.E., Barber J., Hope A., Wilson A.J., Gubbins S., Henstock M., Frost L., Batten C., Veronesi E., Moffat K., et al. Using shared needles for subcutaneous inoculation can transmit bluetongue virus mechanically between ruminant hosts. Sci Rep. 2016;6:20627. doi: 10.1038/srep20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drolet B.S., Reister L.M., Lehiy C.J., Van Rijn P.A., Bowen R.A. Effect of Culicoides sonorensis salivary proteins on clinical disease outcome in experimental bluetongue virus serotype 8 infection of Dorset sheep. Vet. Ital. 2015;51:379–384. doi: 10.12834/VetIt.496.2398.1. [DOI] [PubMed] [Google Scholar]
- 20.Bréard E., Belbis G., Viarouge C., Nomikou K., Haegeman A., De Clercq K., Hudelet P., Hamers C., Moreau F., Lilin T., et al. Evaluation of adaptive immune responses and heterologous protection induced by inactivated bluetongue virus vaccines. Vaccine. 2015;33:512–518. doi: 10.1016/j.vaccine.2014.11.053. [DOI] [PubMed] [Google Scholar]
- 21.Kesik-Maliszewska J., Pomorska-Mol M., Collins A.B., Rola J., Larska M. Potential use of hematological and acute phase protein parameters in the diagnosis of acute Schmallenberg virus infection in experimentally infected calves. Comp. Immunol. Microbiol. Infect. Dis. 2019;64:146–152. doi: 10.1016/j.cimid.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Konig P., Wernike K., Hechinger S., Tauscher K., Breithaupt A., Beer M. Fetal infection with Schmallenberg virus—An experimental pathogenesis study in pregnant cows. Transbound. Emerg Dis. 2019;66:454–462. doi: 10.1111/tbed.13045. [DOI] [PubMed] [Google Scholar]
- 23.Endalew A.D., Morozov I., Davis A.S., Gaudreault N.N., Wernike K., Bawa B., Ruder M.G., Drolet B.S., McVey D.S., Shivanna V., et al. Virological and Serological Responses of Sheep and Cattle to Experimental Schmallenberg Virus Infection. Vector-Borne Zoonotic Dis. 2018 doi: 10.1089/vbz.2018.2297. [DOI] [PubMed] [Google Scholar]
- 24.Laloy E., Bréard E., Trapp S., Pozzi N., Riou M., Barc C., Breton S., Delaunay R., Cordonnier N., Chateau-Joubert S., et al. Fetopathic effects of experimental Schmallenberg virus infection in pregnant goats. Vet Microbiol. 2017;211:141–149. doi: 10.1016/j.vetmic.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Prieto V., Kukielka D., Mourino M., Paradell H., Plaja L., Urniza A., Sanchez-Vizcaino J.M. Natural Immunity of Sheep and Lambs Against the Schmallenberg Virus Infection. Transbound. Emerg. Dis. 2016;63:e220–e228. doi: 10.1111/tbed.12256. [DOI] [PubMed] [Google Scholar]
- 26.Poskin A., Verite S., Comtet L., Van der Stede Y., Cay B., De Regge N. Persistence of the protective immunity and kinetics of the isotype specific antibody response against the viral nucleocapsid protein after experimental Schmallenberg virus infection of sheep. Vet Res. 2015;46:119. doi: 10.1186/s13567-015-0260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinelle L., Poskin A., Dal Pozzo F., De Regge N., Cay B., Saegerman C. Experimental Infection of Sheep at 45 and 60 Days of Gestation with Schmallenberg Virus Readily Led to Placental Colonization without Causing Congenital Malformations. PLoS ONE. 2015;10:e0139375. doi: 10.1371/journal.pone.0139375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinelle L., Poskin A., Dal Pozzo F., Mostin L., Van Campe W., Cay A.B., De Regge N., Saegerman C. Three Different Routes of Inoculation for Experimental Infection with Schmallenberg Virus in Sheep. Transbound. Emerg. Dis. 2017;64:305–308. doi: 10.1111/tbed.12356. [DOI] [PubMed] [Google Scholar]
- 29.Laloy E., Riou M., Barc C., Belbis G., Bréard E., Breton S., Cordonnier N., Crochet D., Delaunay R., Moreau J., et al. Schmallenberg virus: Experimental infection in goats and bucks. BMC Vet. Res. 2015;11:221. doi: 10.1186/s12917-015-0516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poskin A., Martinelle L., Mostin L., Van Campe W., Dal Pozzo F., Saegerman C., Cay A.B., De Regge N. Dose-dependent effect of experimental Schmallenberg virus infection in sheep. Vet. J. 2014;201:419–422. doi: 10.1016/j.tvjl.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Pourianfar H.R., Javadi A., Grollo L. A colorimetric-based accurate method for the determination of enterovirus 71 titer. Indian, J. Virol. 2012;23:303–310. doi: 10.1007/s13337-012-0105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinelle L., Dal Pozzo F., Sarradin P., De Leeuw I., De Clercq K., Thys C., Thiry E., Saegerman C. Pulmonary artery haemorrhage in newborn calves following bluetongue virus serotype 8 experimental infections of pregnant heifers. Vet. Microbiol. 2013;167:250–259. doi: 10.1016/j.vetmic.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Martinelle L., Dal Pozzo F., Sarradin P., De Leeuw I., De Clercq K., Thys C., Ziant D., Thiry E., Saegerman C. Two alternative inocula to reproduce bluetongue virus serotype 8 disease in calves. Vaccine. 2011;29:3600–3609. doi: 10.1016/j.vaccine.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 34.Dal Pozzo F., Martinelle L., Thys C., Sarradin P., De Leeuw I., Van Campe W., De Clercq K., Thiry E., Saegerman C. Experimental co-infections of calves with bluetongue virus serotypes 1 and 8. Vet. Microbiol. 2013;165:167–172. doi: 10.1016/j.vetmic.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Moulin V., Noordegraaf C.V., Makoschey B., van der Sluijs M., Veronesi E., Darpel K., Mertens P.P., de Smit H. Clinical disease in sheep caused by bluetongue virus serotype 8, and prevention by an inactivated vaccine. Vaccine. 2012;30:2228–2235. doi: 10.1016/j.vaccine.2011.11.100. [DOI] [PubMed] [Google Scholar]
- 36.Anderson J., Hagglund S., Bréard E., Riou M., Zohari S., Comtet L., Olofson A.S., Gelineau R., Martin G., Elvander M., et al. Strong protection induced by an experimental DIVA subunit vaccine against bluetongue virus serotype 8 in cattle. Vaccine. 2014;32:6614–6621. doi: 10.1016/j.vaccine.2014.09.066. [DOI] [PubMed] [Google Scholar]
- 37.Domingo E., Holland J.J. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 1997;51:151–178. doi: 10.1146/annurev.micro.51.1.151. [DOI] [PubMed] [Google Scholar]
- 38.Bonneau K.R., Mullens B.A., MacLachlan N.J. Occurrence of genetic drift and founder effect during quasispecies evolution of the VP2 and NS3/NS3A genes of bluetongue virus upon passage between sheep, cattle, and Culicoides sonorensis. J. Virol. 2001;75:8298–8305. doi: 10.1128/JVI.75.17.8298-8305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biebricher C.K., Eigen M. Current Topics in Microbiology and Immunology. Volume 299. Springer; Berlin/Heidelberg, Germany: 2006. What is a quasispecies? pp. 1–31. [DOI] [PubMed] [Google Scholar]
- 40.Domingo E., Escarmis C., Sevilla N., Moya A., Elena S.F., Quer J., Novella I.S., Holland J.J. Basic concepts in RNA virus evolution. FASEB J. 1996;10:859–864. doi: 10.1096/fasebj.10.8.8666162. [DOI] [PubMed] [Google Scholar]
- 41.Fischer M., Hoffmann B., Goller K.V., Hoper D., Wernike K., Beer M. A mutation ‘hot spot’ in the Schmallenberg virus M segment. J. Gen. Virol. 2013;94:1161–1167. doi: 10.1099/vir.0.049908-0. [DOI] [PubMed] [Google Scholar]
- 42.Caporale M., Di Gialleonorado L., Janowicz A., Wilkie G., Shaw A., Savini G., Van Rijn P.A., Mertens P., Di Ventura M., Palmarini M. Virus and host factors affecting the clinical outcome of bluetongue virus infection. J. Virol. 2014;88:10399–10411. doi: 10.1128/JVI.01641-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darpel K.E., Langner K.F., Nimtz M., Anthony S.J., Brownlie J., Takamatsu H.H., Mellor P.S., Mertens P.P. Saliva proteins of vector Culicoides modify structure and infectivity of bluetongue virus particles. PLoS ONE. 2011;6:e17545. doi: 10.1371/journal.pone.0017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eschbaumer M., Wackerlin R., Rudolf M., Keller M., Konig P., Zemke J., Hoffmann B., Beer M. Infectious blood or culture-grown virus: A comparison of bluetongue virus challenge models. Vet. Microbiol. 2010;146:150–154. doi: 10.1016/j.vetmic.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 45.OIE . Bluetongue. In: Health WOfA, editor. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; Paris, France: 2014. pp. 1–18. [Google Scholar]
- 46.Wernike K., Eschbaumer M., Breithaupt A., Hoffmann B., Beer M. Schmallenberg virus challenge models in cattle: Infectious serum or culture-grown virus? Vet. Res. 2012;43:84. doi: 10.1186/1297-9716-43-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wernike K., Hoffmann B., Bréard E., Botner A., Ponsart C., Zientara S., Lohse L., Pozzi N., Viarouge C., Sarradin P., et al. Schmallenberg virus experimental infection of sheep. Vet. Microbiol. 2013;166:461–466. doi: 10.1016/j.vetmic.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 48.Varela M., Schnettler E., Caporale M., Murgia C., Barry G., McFarlane M., McGregor E., Piras I.M., Shaw A., Lamm C., et al. Schmallenberg virus pathogenesis, tropism and interaction with the innate immune system of the host. PLOS Pathog. 2013;9:e1003133. doi: 10.1371/journal.ppat.1003133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraatz F., Wernike K., Hechinger S., Konig P., Granzow H., Reimann I., Beer M. Deletion mutants of Schmallenberg virus are avirulent and protect from virus challenge. J. Virol. 2015;89:1825–1837. doi: 10.1128/JVI.02729-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofmann M.A., Mader M., Fluckiger F., Renzullo S. Genetic stability of Schmallenberg virus in vivo during an epidemic, and in vitro, when passaged in the highly susceptible porcine SK-6 cell line. Vet. Microbiol. 2015;176:97–108. doi: 10.1016/j.vetmic.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venter G.J., Mellor P.S., Wright I., Paweska J.T. Replication of live-attenuated vaccine strains of bluetongue virus in orally infected South African Culicoides species. Med. Vet. Entomol. 2007;21:239–247. doi: 10.1111/j.1365-2915.2007.00687.x. [DOI] [PubMed] [Google Scholar]
- 52.Horne K.M., Vanlandingham D.L. Bunyavirus-vector interactions. Viruses. 2014;6:4373–4397. doi: 10.3390/v6114373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Coupanec A., Babin D., Fiette L., Jouvion G., Ave P., Misse D., Bouloy M., Choumet V. Aedes mosquito saliva modulates Rift Valley fever virus pathogenicity. PLOS Neglected Trop. Dis. 2013;7:e2237. doi: 10.1371/journal.pntd.0002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dal Pozzo F., De Clercq K., Guyot H., Vandemeulebroucke E., Sarradin P., Vandenbussche F., Thiry E., Saegerman C. Experimental reproduction of bluetongue virus serotype 8 clinical disease in calves. Vet. Microbiol. 2009;136:352–358. doi: 10.1016/j.vetmic.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Nicolas J.F., Guy B. Intradermal, epidermal and transcutaneous vaccination: From immunology to clinical practice. Expert Rev. Vaccines. 2008;7:1201–1214. doi: 10.1586/14760584.7.8.1201. [DOI] [PubMed] [Google Scholar]
- 56.Umeshappa C.S., Singh K.P., Channappanavar R., Sharma K., Nanjundappa R.H., Saxena M., Singh R., Sharma A.K. A comparison of intradermal and intravenous inoculation of bluetongue virus serotype 23 in sheep for clinico-pathology, and viral and immune responses. Vet. Immunol. Immunopathol. 2011;141:230–238. doi: 10.1016/j.vetimm.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Melzi E., Caporale M., Rocchi M., Martin V., Gamino V., di Provvido A., Marruchella G., Entrican G., Sevilla N., Palmarini M. Follicular dendritic cell disruption as a novel mechanism of virus-induced immunosuppression. Proc. Natl. Acad. Sci. USA. 2016;113:E6238–E6247. doi: 10.1073/pnas.1610012113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Sluijs M., Timmermans M., Moulin V., Noordegraaf C.V., Vrijenhoek M., Debyser I., de Smit A.J., Moormann R. Transplacental transmission of Bluetongue virus serotype 8 in ewes in early and mid gestation. Vet. Microbiol. 2011;149:113–125. doi: 10.1016/j.vetmic.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 59.van der Sluijs M.T., Schroer-Joosten D.P., Fid-Fourkour A., Vrijenhoek M.P., Debyser I., Moulin V., Moormann R.J., de Smit A.J. Transplacental transmission of Bluetongue virus serotype 1 and serotype 8 in sheep: Virological and pathological findings. PLoS ONE. 2013;8:e81429. doi: 10.1371/journal.pone.0081429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Batten C.A., Henstock M.R., Steedman H.M., Waddington S., Edwards L., Oura C.A. Bluetongue virus serotype 26: Infection kinetics, pathogenesis and possible contact transmission in goats. Vet. Microbiol. 2013;162:62–67. doi: 10.1016/j.vetmic.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 61.Batten C., Darpel K., Henstock M., Fay P., Veronesi E., Gubbins S., Graves S., Frost L., Oura C. Evidence for transmission of bluetongue virus serotype 26 through direct contact. PLoS ONE. 2014;9:e96049. doi: 10.1371/journal.pone.0096049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alexandersen S., Quan M., Murphy C., Knight J., Zhang Z. Studies of quantitative parameters of virus excretion and transmission in pigs and cattle experimentally infected with foot-and-mouth disease virus. J. Comp. Pathol. 2003;129:268–282. doi: 10.1016/S0021-9975(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 63.Howey R., Quan M., Savill N.J., Matthews L., Alexandersen S., Woolhouse M. Effect of the initial dose of foot-and-mouth disease virus on the early viral dynamics within pigs. J. R. Soc. Interface. 2009;6:835–847. doi: 10.1098/rsif.2008.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quan M., Murphy C.M., Zhang Z., Alexandersen S. Determinants of early foot-and-mouth disease virus dynamics in pigs. J. Comp. Pathol. 2004;131:294–307. doi: 10.1016/j.jcpa.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Di Gialleonardo L., Migliaccio P., Teodori L., Savini G. The length of BTV-8 viraemia in cattle according to infection doses and diagnostic techniques. Res. Vet. Sci. 2011;91:316–320. doi: 10.1016/j.rvsc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 66.Pages N., Bréard E., Urien C., Talavera S., Viarouge C., Lorca-Oro C., Jouneau L., Charley B., Zientara S., Bensaid A., et al. Culicoides midge bites modulate the host response and impact on bluetongue virus infection in sheep. PLoS ONE. 2014;9:e83683. doi: 10.1371/journal.pone.0083683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bishop J.V., Mejia J.S., Perez de Leon A.A., Tabachnick W.J., Titus R.G. Salivary gland extracts of Culicoides sonorensis inhibit murine lymphocyte proliferation and no production by macrophages. Am. J. Trop. Med. Hyg. 2006;75:532–536. doi: 10.4269/ajtmh.2006.75.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Limesand K.H., Higgs S., Pearson L.D., Beaty B.J. Effect of mosquito salivary gland treatment on vesicular stomatitis New Jersey virus replication and interferon alpha/beta expression in vitro. J. Med. Entomol. 2003;40:199–205. doi: 10.1603/0022-2585-40.2.199. [DOI] [PubMed] [Google Scholar]
- 69.Boorman J. The maintenance of laboratory colonies of Culicoides variipennis (Coq.), C. nubeculosus (Mg.) and C. riethi Kieff. (Diptera, Ceratopogonidae) Bull. Entomol. Res. 1974;64:371–377. doi: 10.1017/S0007485300031254. [DOI] [Google Scholar]
- 70.Veronesi E., Antony F., Gubbins S., Golding N., Blackwell A., Mertens P.P., Brownlie J., Darpel K.E., Mellor P.S., Carpenter S. Measurement of the infection and dissemination of bluetongue virus in Culicoides biting midges using a semi-quantitative rt-PCR assay and isolation of infectious virus. PLoS ONE. 2013;8:e70800. doi: 10.1371/journal.pone.0070800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Evermann J.F., McKeirnan A.J., Wilbur L.A., Levings R.L., Trueblood E.S., Baldwin T.J., Hughbanks F.G. Canine fatalities associated with the use of a modified live vaccine administered during late stages of pregnancy. J. Vet. Diagn. Investig. 1994;6:353–357. doi: 10.1177/104063879400600312. [DOI] [PubMed] [Google Scholar]
- 72.Wilbur L.A., Evermann J.F., Levings R.L., Stoll I.R., Starling D.E., Spillers C.A., Gustafson G.A., McKeirnan A.J. Abortion and death in pregnant bitches associated with a canine vaccine contaminated with bluetongue virus. J. Am. Vet. Med. Assoc. 1994;204:1762–1765. [PubMed] [Google Scholar]
- 73.Bréard E., Belbis G., Hamers C., Moulin V., Lilin T., Moreau F., Millemann Y., Montange C., Sailleau C., Durand B., et al. Evaluation of humoral response and protective efficacy of two inactivated vaccines against bluetongue virus after vaccination of goats. Vaccine. 2011;29:2495–2502. doi: 10.1016/j.vaccine.2010.12.105. [DOI] [PubMed] [Google Scholar]
- 74.Eschbaumer M., Wackerlin R., Savini G., Zientara S., Sailleau C., Bréard E., Beer M., Hoffmann B. Contamination in bluetongue virus challenge experiments. Vaccine. 2011;29:4299–4301. doi: 10.1016/j.vaccine.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 75.Rasmussen L.D., Savini G., Lorusso A., Bellacicco A., Palmarini M., Caporale M., Rasmussen T.B., Belsham G.J., Botner A. Transplacental transmission of field and rescued strains of BTV-2 and BTV-8 in experimentally infected sheep. Vet. Res. 2013;44:75. doi: 10.1186/1297-9716-44-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vandenbussche F., Sailleau C., Rosseel T., Desprat A., Viarouge C., Richardson J., Eschbaumer M., Hoffmann B., De Clercq K., Bréard E., et al. Full-Genome Sequencing of Four Bluetongue Virus Serotype 11 Viruses. Transbound. Emerg. Dis. 2015;62:565–571. doi: 10.1111/tbed.12178. [DOI] [PubMed] [Google Scholar]
- 77.van Rijn P.A., Geurts Y., van der Spek A.N., Veldman D., van Gennip R.G. Bluetongue virus serotype 6 in Europe in 2008-Emergence and disappearance of an unexpected non-virulent BTV. Vet. Microbiol. 2012;158:23–32. doi: 10.1016/j.vetmic.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 78.Orlowska A., Trebas P., Smreczak M., Marzec A., Zmudzinski J.F. First detection of bluetongue virus serotype 14 in Poland. Arch. Virol. 2016;161:1969–1972. doi: 10.1007/s00705-016-2857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilbert-Barness E. Teratogenic causes of malformations. Ann. Clin. Lab. Sci. 2010;40:99–114. [PubMed] [Google Scholar]
- 80.MacLachlan N.J., Conley A.J., Kennedy P.C. Bluetongue and equine viral arteritis viruses as models of virus-induced fetal injury and abortion. Anim. Reprod. Sci. 2000;60–61:643–651. doi: 10.1016/S0378-4320(00)00105-6. [DOI] [PubMed] [Google Scholar]
- 81.Maclachlan N.J., Osburn B.I. Teratogenic bluetongue and related orbivirus infections in pregnant ruminant livestock: Timing and pathogen genetics are critical. Curr. Opin. Virol. 2017;27:31–35. doi: 10.1016/j.coviro.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Doceul V., Lara E., Sailleau C., Belbis G., Richardson J., Bréard E., Viarouge C., Dominguez M., Hendrikx P., Calavas D., et al. Epidemiology, molecular virology and diagnostics of Schmallenberg virus, an emerging orthobunyavirus in Europe. Vet. Res. 2013;44:31. doi: 10.1186/1297-9716-44-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garigliany M.M., Bayrou C., Kleijnen D., Cassart D., Desmecht D. Schmallenberg virus in domestic cattle, Belgium, 2012. Emerg. Infect. Dis. 2012;18:1512–1514. doi: 10.3201/eid1809.120716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parsonson I.M., McPhee D.A., Della-Porta A.J., McClure S., McCullagh P. Transmission of Akabane virus from the ewe to the early fetus (32 to 53 days) J. Comp. Pathol. 1988;99:215–227. doi: 10.1016/0021-9975(88)90073-4. [DOI] [PubMed] [Google Scholar]
- 85.Charles J.A. Akabane virus. Vet. Clin. N. Am. 1994;10:525–546. doi: 10.1016/S0749-0720(15)30537-5. [DOI] [PubMed] [Google Scholar]
- 86.King G.J., Atkinson B.A., Robertson H.A. Development of the bovine placentome during the second month of gestation. Reproduction. 1979;55:173–180. doi: 10.1530/jrf.0.0550173. [DOI] [PubMed] [Google Scholar]
- 87.Assis Neto A.C., Pereira F.T., Santos T.C., Ambrosio C.E., Leiser R., Miglino M.A. Morpho-physical recording of bovine conceptus (Bos indicus) and placenta from days 20 to 70 of pregnancy. Reprod. Domest. Anim. 2010;45:760–772. doi: 10.1111/j.1439-0531.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 88.Spencer T.E., Johnson G.A., Bazer F.W., Burghardt R.C. Implantation mechanisms: Insights from the sheep. Reproduction. 2004;128:657–668. doi: 10.1530/rep.1.00398. [DOI] [PubMed] [Google Scholar]
- 89.Bryden M.M., Evans H.E., Binns W. Embryology of the sheep. I. Extraembryonic membranes and the development of body form. J. Morphol. 1972;138:169–185. doi: 10.1002/jmor.1051380204. [DOI] [PubMed] [Google Scholar]
- 90.Evans H.E., Sack W.O. Prenatal development of domestic and laboratory mammals: Growth curves, external features and selected references. Anat. Histol. Embryol. 1973;2:11–45. doi: 10.1111/j.1439-0264.1973.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 91.Jordan R.K. Development of sheep thymus in relation to in utero thymectomy experiments. Eur. J. Immunol. 1976;6:693–698. doi: 10.1002/eji.1830061007. [DOI] [PubMed] [Google Scholar]
- 92.Khaksary-Mahabady M., Khazaeel K., Pourmahdi Borujeni M., Yazdanjoo B. Morphometric development of sheep (Ovis Aries) lymph nodes in fetal period. Vet. Res. Forum. 2018;9:121–128. doi: 10.30466/VRF.2018.30833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maddox J.F., Mackay C.R., Brandon M.R. Ontogeny of ovine lymphocytes. II. An immunohistological study on the development of T lymphocytes in the sheep fetal spleen. Immunology. 1987;62:107–112. [PMC free article] [PubMed] [Google Scholar]
- 94.Szabo K.T. Congenital Malformations in Laboratory and Farm Animals. Academic Press; Cambridge, MA, USA: 1989. [Google Scholar]
- 95.Coppock R.W., Dziwenka M.M. Reproductive and Developmental Toxicology. 2nd ed. Elsevier; Atlanta, GA, USA: 2017. Chapter 72—Teratogenesis in Livestock; pp. 1391–1408. [DOI] [Google Scholar]
- 96.Schultz R.D., Dunne H.W., Heist C.E. Ontogeny of the bovine immune response. Infect. Immun. 1973;7:981–991. doi: 10.1128/iai.7.6.981-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silverstein A.M., Uhr J.W., Kraner K.L., Lukes R.J. Fetal response to antigenic stimulus. II. Antibody production by the fetal lamb. J. Exp. Med. 1963;117:799–812. doi: 10.1084/jem.117.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fahey K.J., Morris B. Humoral immune responses in foetal sheep. Immunology. 1978;35:651–661. [PMC free article] [PubMed] [Google Scholar]
- 99.De Clercq K., De Leeuw I., Verheyden B., Vandemeulebroucke E., Vanbinst T., Herr C., Meroc E., Bertels G., Steurbaut N., Miry C., et al. Transplacental infection and apparently immunotolerance induced by a wild-type bluetongue virus serotype 8 natural infection. Transbound. Emerg. Dis. 2008;55:352–359. doi: 10.1111/j.1865-1682.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- 100.De Regge N., van den Berg T., Georges L., Cay B. Diagnosis of Schmallenberg virus infection in malformed lambs and calves and first indications for virus clearance in the fetus. Vet. Microbiol. 2013;162:595–600. doi: 10.1016/j.vetmic.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 101.Maclachlan N.J., Drew C.P., Darpel K.E., Worwa G. The pathology and pathogenesis of bluetongue. J. Comp. Pathol. 2009;141:1–16. doi: 10.1016/j.jcpa.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 102.Coetzee P., Stokstad M., Myrmel M., Mutowembwa P., Loken T., Venter E.H., Van Vuuren M. Transplacental infection in goats experimentally infected with a European strain of bluetongue virus serotype 8. Vet. J. 2013;197:335–341. doi: 10.1016/j.tvjl.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 103.Poskin A., Martinelle L., Van der Stede Y., Saegerman C., Cay A.B., De Regge N. Genetically stable infectious Schmallenberg virus persists in foetal envelopes of pregnant ewes. J. Gen. Virol. 2017;98:1630–1635. doi: 10.1099/jgv.0.000841. [DOI] [PubMed] [Google Scholar]
- 104.Consortium E. Schmallenberg Virus Technical and Scientific Studies Final Report. Central Veterinary Institute; Wageningen, The Netherlands: 2014. p. 67. [Google Scholar]
- 105.Santman-Berends I.M., van Wuijckhuise L., Vellema P., van Rijn P.A. Vertical transmission of bluetongue virus serotype 8 virus in Dutch dairy herds in 2007. Vet. Microbiol. 2010;141:31–35. doi: 10.1016/j.vetmic.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 106.van Wuijckhuise L., Vellema P., Pelgrim W., Tolboom R., Hans M., van Rijn P. Bluetongue virus serotype 8 in healthy young calves. Tijdschr. Diergeneeskd. 2008;133:992–994. [PubMed] [Google Scholar]
- 107.Darpel K.E., Batten C.A., Veronesi E., Williamson S., Anderson P., Dennison M., Clifford S., Smith C., Philips L., Bidewell C., et al. Transplacental transmission of bluetongue virus 8 in cattle, UK. Emerg. Infect. Dis. 2009;15:2025–2028. doi: 10.3201/eid1512.090788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Desmecht D., Bergh R.V., Sartelet A., Leclerc M., Mignot C., Misse F., Sudraud C., Berthemin S., Jolly S., Mousset B., et al. Evidence for transplacental transmission of the current wild-type strain of bluetongue virus serotype 8 in cattle. Vet. Rec. 2008;163:50–52. doi: 10.1136/vr.163.2.50. [DOI] [PubMed] [Google Scholar]
- 109.Kirkland P.D., Hawkes R.A. A comparison of laboratory and ‘wild’ strains of bluetongue virus--is there any difference and does it matter? Vet. Ital. 2004;40:448–455. [PubMed] [Google Scholar]
- 110.Osburn B.I., Johnson R.T., Silverstein A.M., Prendergast R.A., Jochim M.M., Levy S.E. Experimental viral-induced congenital encephalopathies. II. The pathogenesis of bluetongue vaccine virus infection in fetal lambs. Lab. Investig. 1971;25:206–210. [PubMed] [Google Scholar]
- 111.Anderson C.K., Jensen R. Pathologic changes in placentas of ewes inoculated with bluetongue virus. Am. J. Vet. Res. 1969;30:987–989. [PubMed] [Google Scholar]
- 112.Belbis G., Bréard E., Cordonnier N., Moulin V., Desprat A., Sailleau C., Viarouge C., Doceul V., Zientara S., Millemann Y. Evidence of transplacental transmission of bluetongue virus serotype 8 in goats. Vet. Microbiol. 2013;166:394–404. doi: 10.1016/j.vetmic.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 113.Kirkland P.D., Barry R.D., Harper P.A., Zelski R.Z. The development of Akabane virus-induced congenital abnormalities in cattle. Vet. Rec. 1988;122:582–586. doi: 10.1136/vr.122.24.582. [DOI] [PubMed] [Google Scholar]
- 114.Saegerman C., Bolkaerts B., Baricalla C., Raes M., Wiggers L., de Leeuw I., Vandenbussche F., Zimmer J.Y., Haubruge E., Cassart D., et al. The impact of naturally-occurring, trans-placental bluetongue virus serotype-8 infection on reproductive performance in sheep. Vet. J. 2011;187:72–80. doi: 10.1016/j.tvjl.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 115.MacLachlan N.J., Osburn B.I., Stott J.L., Ghalib H.W. Orbivirus infection of the bovine fetus. Prog. Clin. Boil. Res. 1985;178:79–84. [PubMed] [Google Scholar]
- 116.Vercauteren G., Miry C., Vandenbussche F., Ducatelle R., Van der Heyden S., Vandemeulebroucke E., De Leeuw I., Deprez P., Chiers K., De Clercq K. Bluetongue virus serotype 8-associated congenital hydranencephaly in calves. Transbound. Emerg. Dis. 2008;55:293–298. doi: 10.1111/j.1865-1682.2008.01034.x. [DOI] [PubMed] [Google Scholar]
- 117.Richardson C., Taylor W.P., Terlecki S., Gibbs E.P. Observations on transplacental infection with bluetongue virus in sheep. Am. J. Vet. Res. 1985;46:1912–1922. [PubMed] [Google Scholar]
- 118.Savini G., Lorusso A., Paladini C., Migliaccio P., Di Gennaro A., Di Provvido A., Scacchia M., Monaco F. Bluetongue serotype 2 and 9 modified live vaccine viruses as causative agents of abortion in livestock: A retrospective analysis in Italy. Transbound. Emerg. Dis. 2014;61:69–74. doi: 10.1111/tbed.12004. [DOI] [PubMed] [Google Scholar]
- 119.Waldvogel A.S., Anderson G.A., Phillips D.L., Osburn B.I. Infection of bovine fetuses at 120 days’ gestation with virulent and avirulent strains of bluetongue virus serotype 11. Comp. Immunol. Microbiol. Infect. Dis. 1992;15:53–63. doi: 10.1016/0147-9571(92)90102-W. [DOI] [PubMed] [Google Scholar]
- 120.McKercher D.G., Saito J.K., Singh K.V. Serologic evidence of an etiologic role for bluetongue virus in hydranencephaly of calves. J. Am. Vet. Med. Assoc. 1970;156:1044–1047. [PubMed] [Google Scholar]
- 121.Wernike K., Elbers A., Beer M. Schmallenberg virus infection. Rev. Sci. Tech. 2015;34:363–373. doi: 10.20506/rst.34.2.2363. [DOI] [PubMed] [Google Scholar]
- 122.Veldhuis A.M., Carp-van Dijken S., van Wuijckhuise L., Witteveen G., van Schaik G. Schmallenberg virus in Dutch dairy herds: Potential risk factors for high within-herd seroprevalence and malformations in calves, and its impact on productivity. Vet. Microbiol. 2014;168:281–293. doi: 10.1016/j.vetmic.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 123.Dominguez M., Gache K., Touratier A., Perrin J.B., Fediaevsky A., Collin E., Bréard E., Sailleau C., Viarouge C., Zanella G., et al. Spread and impact of the Schmallenberg virus epidemic in France in 2012–2013. BMC Vet. Res. 2014;10:248. doi: 10.1186/s12917-014-0248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dominguez M., Hendrikx P., Zientara S., Calavas D., Jay M., Touratier A., Languille J., Fediaevsky A. Preliminary estimate of Schmallenberg virus infection impact in sheep flocks—France. Vet. Rec. 2012;171:426. doi: 10.1136/vr.100883. [DOI] [PubMed] [Google Scholar]
- 125.Saegerman C., Martinelle L., Dal Pozzo F., Kirschvink N. Preliminary survey on the impact of Schmallenberg virus on sheep flocks in South of Belgium. Transbound. Emerg. Dis. 2014;61:469–472. doi: 10.1111/tbed.12047. [DOI] [PubMed] [Google Scholar]
- 126.Agerholm J.S., Hewicker-Trautwein M., Peperkamp K., Windsor P.A. Virus-induced congenital malformations in cattle. Acta Vet. Scand. 2015;57:54. doi: 10.1186/s13028-015-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martinelle L., Dal Pozzo F., Kirschvink N., De la Grandiere M.A., Thiry E., Saegerman C. Le virus Schmallenberg ou l’émergence du premier Orthobunyavirus du sérogroupe Simbu en Europe. Ann. Med. Vet. 2012;156:7–24. [Google Scholar]
- 128.Afonso A., Abrahantes J.C., Conraths F., Veldhuis A., Elbers A., Roberts H., Van der Stede Y., Meroc E., Gache K., Richardson J. The Schmallenberg virus epidemic in Europe-2011-2013. Prev. Vet. Med. 2014;116:391–403. doi: 10.1016/j.prevetmed.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 129.Dixon A.B., Knights M., Winkler J.L., Marsh D.J., Pate J.L., Wilson M.E., Dailey R.A., Seidel G., Inskeep E.K. Patterns of late embryonic and fetal mortality and association with several factors in sheep. J. Anim. Sci. 2007;85:1274–1284. doi: 10.2527/jas.2006-129. [DOI] [PubMed] [Google Scholar]
- 130.Diskin M.G., Morris D.G. Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 2008;43(Suppl. 2):260–267. doi: 10.1111/j.1439-0531.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- 131.Geary T. Management Strategies to Reduce Embryonic Loss; Proceedings of the Range Beef Cow Symposium; Miles City, MT, USA. 6–8 December 2005. [Google Scholar]
- 132.Nusinovici S., Seegers H., Joly A., Beaudeau F., Fourichon C. Quantification and at-risk period of decreased fertility associated with exposure to bluetongue virus serotype 8 in naive dairy herds. J. Dairy Sci. 2012;95:3008–3020. doi: 10.3168/jds.2011-4799. [DOI] [PubMed] [Google Scholar]
- 133.Santman-Berends I.M., Hage J.J., van Rijn P.A., Stegeman J.A., van Schaik G. Bluetongue virus serotype 8 (BTV-8) infection reduces fertility of Dutch dairy cattle and is vertically transmitted to offspring. Theriogenology. 2010;74:1377–1384. doi: 10.1016/j.theriogenology.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 134.Lechner I., Wuthrich M., Meylan M., van den Borne B.H.P., Schupbach-Regula G. Association of clinical signs after acute Schmallenberg virus infection with milk production and fertility in Swiss dairy cows. Prev. Vet. Med. 2017;146:121–129. doi: 10.1016/j.prevetmed.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 135.Belgian Biosafety Server. [(accessed on 15 August 2018)]; Available online: https://www.biosafety.be/content/tools-belgian-classification-micro-organisms-based-their-biological-risks.
- 136.Spencer T.E., Forde N., Lonergan P. Insights into conceptus elongation and establishment of pregnancy in ruminants. Reprod. Fertil. Dev. 2016;29:84–100. doi: 10.1071/RD16359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.