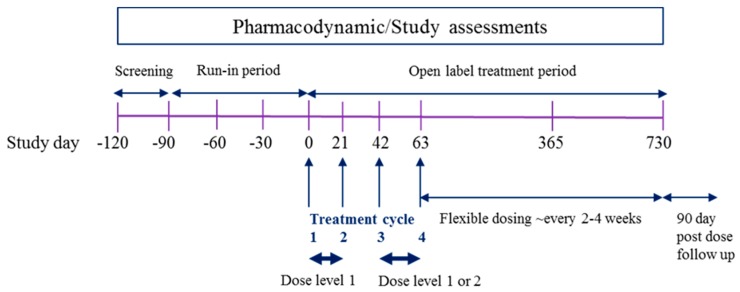
Figure 2.
Study Design Schematic. The overall study duration will be 31 months, comprising 28 days screening, 90 days run-in, 24 months open label treatment and 90 days post-dose follow-up. The first 4 treatment cycles will be administered every 21 days, with patients receiving Dose Level 1 for the first 2 treatment cycles. If metabolic correction is not achieved, the subsequent 2 treatment cycles will be administered at Dose Level 2. From treatment day 78, a flexible dosing approach will be employed to achieve metabolic correction, where administration will be at Dose Levels 1, 2 or 3, once every 2–4 weeks ± 2 days until the end of the study.