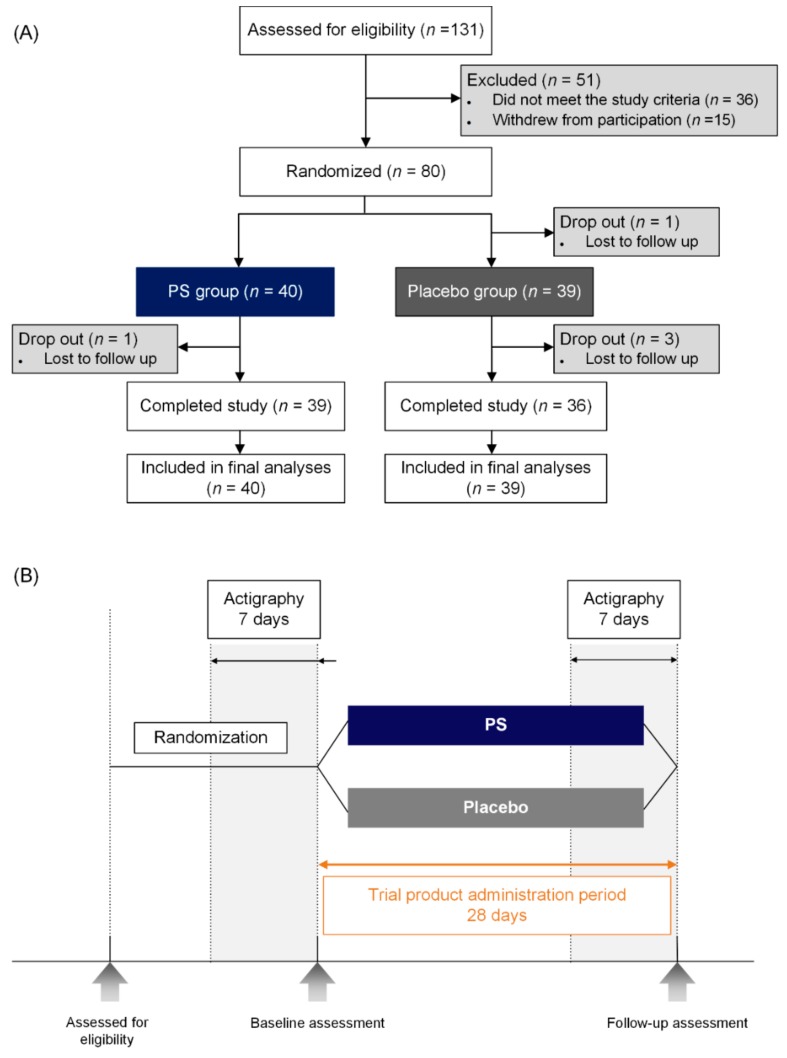
Figure 1.
(A) Flowchart of the clinical trial. Eighty participants were enrolled and randomly assigned into one of two groups: 40 participants received placebo (Placebo group) and 40 received PS (PS group). (B) Study procedure. After randomization, the baseline evaluation was performed, which included the assessment of the Athens Insomnia Scale (AIS), objective sleep measures acquired using actigraphy, and multimodal brain MRI data. A follow-up visit was scheduled upon completion of the administration period. PS; Polygonatum sibiricum.