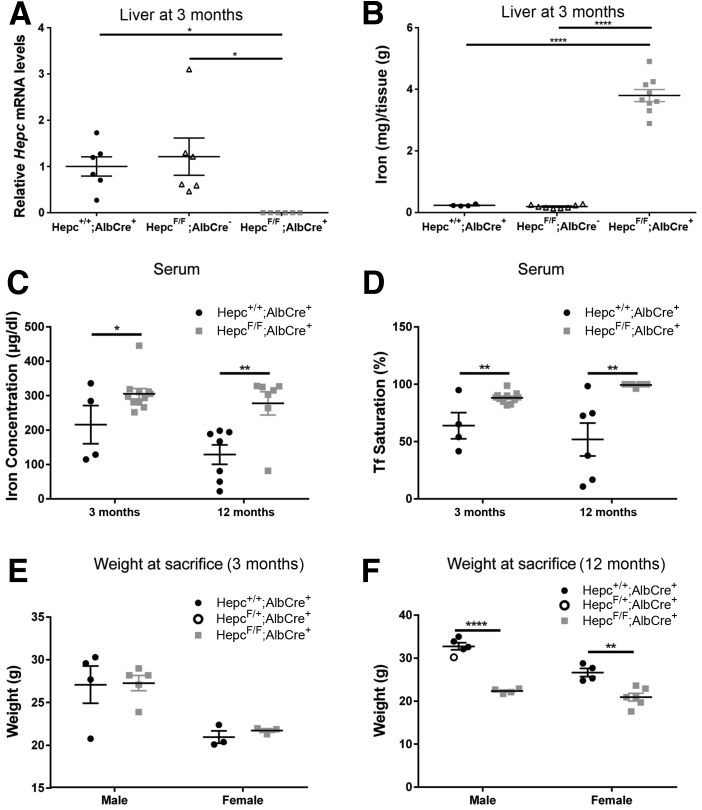
Figure 1.
Validation of LS-HepcKO model. A: Liver Hepc mRNA levels measured by real-time quantitative PCR in LS-HepcKO (Hepcflox/flox;Alb-Cre+) versus controls (Hepc+/+;Alb-Cre+ or Hepcflox/flox;Alb-Cre-) at 3 months. B: Nonheme liver iron quantification (by bathophenanthroline sulfonate assay) in the LS-HepcKO mice compared with controls (Hepc+/+;Alb-Cre+ or Hepcflox/flox;Alb-Cre-) at 3 months. Statistical analysis for liver Hepc mRNA and liver iron quantification was performed using one-way analysis of variance with post hoc pairwise comparisons using the Tukey method. C: Serum iron concentration of LS-HepcKO mice versus controls at 3 and 12 months. D: Serum transferrin (Tf) saturation of LS-HepcKO mice versus controls at 3 and 12 months. For Tf saturation calculation, any value that was calculated to be >100%, because of the presence of nontransferrin bound iron, was recorded as 100% Tf saturation on the graph. E: Body weight of LS-HepcKO versus control mice at 3 months. F: Body weight of LS-HepcKO versus control mice at 12 months. Statistical analysis was performed using two-group, two-sided t-test. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗∗P < 0.0001.