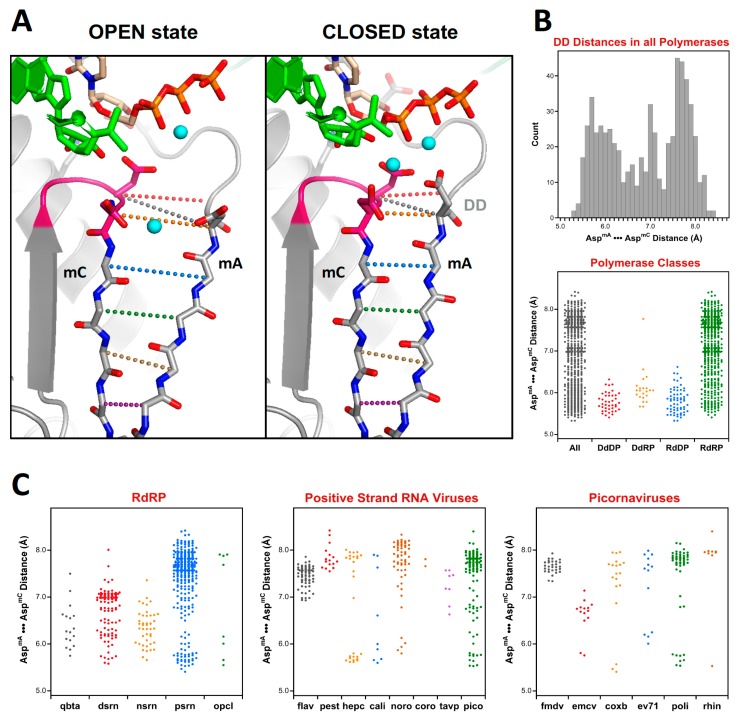
Figure 11.
Analysis of active site closure mechanism in viral polymerases. (A) Comparison of the open and closed states of polio polymerase, where the closed state features a fully hydrogen-bonded anti-parallel β-sheet between motifs A and C, but in the open state the sheet is frayed near the active site. The distance between the two active site aspartates (DD, shown in grey dots) provides a convenient measure of the active site conformation. (B) Histogram of such DD distances for all the polymerases in the superposition set. The bottom panel is a dot plot of the histogram data (All) alongside dot plots that further categorize the distances by polymerase type. (C) A further breakdown of the DD distances observed in all RdRPs, then in the positive strand RNA virus polymerases, and finally in six families of picornaviruses.