Abstract
The Lamiaceae family comprises many flowering plants classified into about 236 genera. The genus Ziziphora is one of the well-known genera of this family and its species are important in different fields of pharmaceutical, chemical, traditional, and folk medicines. The phytochemicals present in Ziziphora include monoterpenic essential oils, triterpenes, and phenolic substances. The aim of this paper was to study the phytochemical profile of Ziziphora taurica subsp. taurica and compare and evaluate the biological activities of its ethyl acetate (ZTT-EtOAc), methanolic (ZTT-MeOH), and aqueous (ZTT-W) extracts based on their enzyme inhibition and antioxidant capacities. Determination of total phenolic (TPC) and total flavonoid (TFC) contents as well as biological activities were determined using spectrophotometric procedures. Subsequently, the individual phenolic compounds were detected by liquid chromatography–electrospray ionization–tandem mass spectrometry (LC–ESI–MS/MS). In total, twenty-two different phenolic compounds were identified, including apigenin, ferulic acid, and luteolin which were the most common. ZTT-MeOH extract showed the best antioxidant activity, whereas ZTT-EtOAc extract was the most effective against tyrosinase and α-amylase. Ziziphora taurica subsp. taurica represents a potential source of natural compounds with positive effects on human health.
Keywords: biological activities, Ziziphora taurica subsp. taurica, total phenolic content, total flavonoid content, apigenin, LC–ESI–MS/MS
1. Introduction
In recent years, the study of natural products for the discovery of new active compounds with beneficial properties for human health is growing worldwide [1]. Plants represent sources of essential oils or secondary metabolites with a wide range of therapeutic action, and using them avoids the side effects typical of synthetic drugs. Consequently, their use has become widespread, above all else, for the sake of human health [2].
Lamiaceae is one of the most important herbal families, well known for their medicinal effects [3]. The prototypical of the Lamiaceae family is represented by the Ziziphora species, and its world population is represented by more than 30 different species inhabiting regions of Asia, Europe, and Africa. However, their largest aggregations are in China and Kazakhstan and in some regions of Afghanistan, Anatolia, Armenia, Caucasia, Iraq, Pakistan, Syria, Turkey, Turkmenistan, and West Siberia. Ziziphora includes annual, perennial, herbaceous, or sub-shrub plants. The leaves are short, petiolate, or sub-sessile. The flowering period is from June to September, depending on environmental conditions [4].
The history of using plants of the genus Ziziphora began in ancient times. The active ingredients of these plants were usually administered as infusions against infections, hemorrhoids, hypertension, and gastrointestinal problems [5]. The first ethnopharmacological reports relate to the use of Ziziphora extracts as a potential medicine for wound healing and edema treatment, and as a potential antipyretic drug [6]. Many of the described species of the genus Ziziphora, in particular Z. clinopodioides L. and Z. tenuior L., were repeatedly prescribed in folk medicine in many countries for the treatment of colds, bronchitis, coughs, headache, diarrhea, nausea, typhus, and even cardiovascular disorders. Some of these species have been recognized not only as having effective anti-inflammatory, tranquilizing, or analgesic properties, but also as aphrodisiacs and flavorings [5].
Previously conducted studies on the phytochemical profile of species belonging to the genus Ziziphora have been mainly focused on the composition of its essential oils. Apart from essential oils, these species are also a rich source of other secondary metabolites, including flavonoids, derivatives of caffeic acid, fatty acids, triterpenes, and sterols [4,7], more than forty natural compounds have been isolated from different Ziziphora species. Among these secondary metabolites, phenolic compounds are certainly the most important due to their positive impact on health, reducing the risk of cardiovascular disease, neurodegenerative disorders, and cancer [8]. One of the best strategies to verify the activity of these compounds is to study their inhibitory capacity against various enzymes such as tyrosinase or α-amylase. Tyrosinase is a key enzyme in melanin biosynthesis that catalyzes two important reactions: the hydroxylation of L-tyrosine to 3,4-dihydroxy-L-phenylalanine (L-DOPA) and the oxidation of L-DOPA to dopaquinone followed by further conversion to melanin. The tyrosinase inhibitor plays an important role in many diseases such as skin cancer or other disorders concerning the hyperpigmentation of melanin [9]. α-Amylase is a hydrolytic enzyme that intervenes in the digestion of carbohydrates. Inhibition of this enzyme in diabetic patients leads to a decrease in plasma glucose levels [10].
Following our research on endemic plants used in traditional and folk medicine which could represent a valid source of bioactive components and their respective biological activities [11,12], the purpose of this research is to study the phytochemical profile of Z. taurica subsp. taurica, as seen in Figure 1, which has not yet been studied, and to assess its biological activity based on evaluations of its antioxidant and enzymatic activities.
Figure 1.
Image of the Z. taurica subsp. taurica.
2. Materials and Methods
2.1. Plant Material
The aerial parts of Ziziphora taurica subsp. taurica (Lamiaceae) were harvested from Nebiler village, Kavaklidere, Mugla-Turkey on 17 June 2018 (1074 m, 37° 27′ 10.7” N 28° 25′ 29.0” E). The species (O.1755) was identified by Dr. Olcay Ceylan and deposited at the Herbarium of University of Mugla Sitki Kocman (Mugla, Turkey). The aerial parts were air-dried in the shade for a few weeks and then cut into small pieces with a laboratory mill before further sample treatments, solvent extractions, and analyses.
2.2. Solvent Extraction
Ethyl acetate (ZTT-EtOAc) and methanol (ZTT-MeOH) extracts from Z. taurica subsp. taurica aerial parts were separately prepared by maceration for 24 h and then concentrated under reduced pressure. The water extract (ZTT-W) was obtained by infusion in boiling deionized water for 15 min, and the obtained solution was lyophilized [10]. Five grams of the roots were mixed with 100 mL of solvent (1:20), and agitation was set to 150 rpm. The obtained extracts were stored at +4 °C for further analysis. Extraction yields are given in Table 1.
Table 1.
Abbreviation, extraction yield, and total phenolic and flavonoid content of the solvent extracts from Z. taurica subsp. taurica x.
| Extracts | Abbreviation | Yield (%) | Total Phenolics (mg GAEs/g extract) | Total Flavonoids (mg QEs/g extract) |
|---|---|---|---|---|
| Ethyl Acetate | ZTT-EtOAc | 3.23 | 34.82 ± 0.79 a | 20.87 ± 1.38 b |
| Methanol | ZTT-MeOH | 8.93 | 27.49 ± 0.74 b | 37.39 ± 2.54 a |
| Water | ZTT-W | 13.69 | 15.10 ± 0.45 c | 7.08 ± 0.58 c |
x Within each column, means sharing the different superscripts show a comparison between the extracts by Tukey’s test at p < 0.05. GAEs and QEs: gallic acid and quercetin equivalents, respectively.
2.3. Chemicals
Gallic acid, (+)-catechin, pyrocatechol, chlorogenic acid, 2,5-dihydroxybenzoic acid, 4-hydroxybenzoic acid, (−)-epicatechin, caffeic acid, syringic acid, vanillin, taxifolin, sinapic acid, p-coumaric acid, ferulic acid, rosmarinic acid, 2-hydroxycinnamic acid, pinoresinol, quercetin, luteolin, and apigenin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Vanillic acid, 3-hydroxybenzoic acid, 3,4-dihydroxyphenylacetic acid, apigenin 7-glucoside, luteolin 7-glucoside, hesperidin, eriodictyol, and kaempferol were obtained from Fluka (St. Louis, MO, USA). Verbascoside, protocatechuic acid, and hyperoside were purchased from HWI Analytik (Ruelzheim, Germany).
2.4. Quantification of Phenolic Compounds in the Extracts
Total phenolic (TPC) and flavonoid (TFC) contents in the extracts were firstly determined spectrophotometrically as gallic acid and quercetin equivalents, respectively [13]. Phenolic composition in the extracts was then detected by liquid chromatography–electrospray tandem mass spectrometry (LC–ESI–MS/MS). Analysis was carried out by the Agilent Technologies 1260 Infinity liquid chromatography system hyphenated to a 6420 Triple Quad mass spectrometer. A Poroshell 120 EC-C18 (100 × 4.6 mm I.D., 2.7 µm) column was used. The mobile phases were 0.1%, v/v formic acid solution (Solvent A) and methanol (Solvent B), in gradient elution. Particularly, the gradient elution profile was: 0.00 min 2% B, 3.00 min 2% B, 6.00 min 25% B, 10.00 min 50% B, 14.00 min 95% B, 17.00 min 95% B, and 17.50 min 2% B. The total run time was 18 min. The column temperature was set at 25 °C. The flow rate was 0.4 mL min−1, and the injection volume was 2.0 μL [14,15]. Furthermore, the tandem MS conditions were an ESI source operated in negative and positive multiple reaction monitoring (MRM) mode, a capillary voltage of −3.5 kV, a gas temperature of 300 °C, a gas flow of 11 L min−1, and a nebulizer pressure of 40 psi. For the quali-quantitative analyses, the single compound was identified by means of retention time, MS, and MS/MS spectra with respect to the standard solution.
2.5. Biological Activity
Biological activities of the extracts were determined by firstly identifying their antioxidant capacity though cupric ion (CUPRAC) and ferric ion (FRAP) reducing power, free radical scavenging activity of DPPH, ABTS, a phosphomolybdenum assay, and ferrous ion chelating [16,17,18,19].
For enzymatic inhibitory activities, the extracts were tested against α-amylase and tyrosinase using the experiment previously reported [13]. In section Supplementary Materials (S1) was given analytical methods applied for polyphenolic composition (TPC, TFC), antioxidant and enzyme inhibitory activities.
The sample concentration, which decreases the initial concentration by 50% for enzyme inhibition, radical scavenging, and metal chelation tests and provides 0.500 absorbance for reducing power and phosphomolybdenum assays, was defined as IC50. The biological activities of the extracts were compared with those of the standards, including Trolox, ethylenediaminetetraacetic acid (disodium salt) (EDTA), kojic acid, and acarbose, used as positive controls. The biological activities of the extracts were also given as mg standard equivalent/g extract.
2.6. Statistical Analysis
Results were illustrated as mean ± standard deviation. Statistical analysis was performed using SPSS software v22.0. Statistical significance was tested by one-way ANOVA (Tukey test). Values were considered significant when p-value was lower than 0.05.
3. Results and Discussion
3.1. Total Phenolic (TPC) and Flavonoid (TFC) Content
Due to the different polarities of phenolic compounds, a single extraction method is not available for these compounds and different extraction solvents (ethyl acetate, methanol, and water) were used in this work. The results obtained for each solvent are shown in Table 1. As reported, the maximum yield from the raw plant material after the extraction procedure was obtained in water (ca. 14%), whereas the lowest yields were observed in ethyl acetate extract (ca. 3%). In the literature, there is no information regarding the effect of different solvents on the extraction of phytochemicals from Ziziphora, but it has been observed in different species [20] that the yields for these compounds are greater in aqueous extracts.
The Ziziphora extract TPC determination was carried out through the Folin–Ciocâlteau assay. The Folin–Ciocâlteau method measures the reduction of the reactant by phenolic compounds through the formation of a complex that can be measured at 750 nm against gallic acid as a standard. In all three extracts analyzed, phenolic compounds and flavonoids were observed. The maximum TPC was registered in the ZTT-EtOAc (34.82 ± 0.79 mg GAEs/g extract), whereas the lowest concentration was present in the ZTT-W (15.10 ± 0.45 mg GAEs/g extract). The results are shown in Table 1.
Results of the total flavonoid content (TFC) of the obtained extracts are also reported in Table 1. The highest amounts were observed for the ZTT-MeOH (37.39 mg QEs/g extract) and ZTT-EtOAc (20.87 mg QEs/g extract), in contrast to ZTT-W, which contains a lower concentration (7.08 mg QEs/g extract). This observation suggests that most of the flavonoids were taken up by the methanol or the ethyl acetate. The findings show that, in all varieties tested, the ethyl acetate and methanol extracts had a higher concentration of flavonoids in comparison to the water extract.
3.2. LC-ESI-MS/MS Analysis
To identify the individual phenolic compounds, a liquid chromatography–electrospray-tandem mass spectrometry analysis method was utilized [14]. In total, in Ziziphora extracts, twenty-five phenolic compounds were identified and reported in Table 2 with the ZTT-MeOH extract containing the highest number of compounds (21 compounds). The most common compounds are apigenin (1475.99 and 1270.90 µg/g extract, respectively, in ZTT-EtOAc extract and in the ZTT-MeOH extract), ferulic acid (1478.13 and 1540.91 µg/g extract, respectively, in ZTT-EtOAc extract and in ZTT-MeOH extract), and luteolin (5347.32 and 5339.91 µg/g extract respectively in ZTT-EtOAc extract and in ZTT-MeOH extract).
Table 2.
Concentration (µg/g extract) and analytical characteristics of selected phytochemicals in the solvent extracts from Z. taurica subsp. taurica x.
| Compound | ZTT-EtOAc | ZTT-MeOH | ZTT-W | Linear Equation | R2 | LOD (μg/L) | LOQ (μg/L) |
|---|---|---|---|---|---|---|---|
| (-)-Epicatechin | nd | nd | nd | y = 9.11x−9.99 | 0.9971 | 1.85 | 6.18 |
| (+)-Catechin | nd | 11.79 ± 0.21 | nd | y = 1.45x+1.95 | 0.9974 | 3.96 | 13.20 |
| 2,5-Dihydroxybenzoic Acid | 15.98 ± 0.84 c | 60.48 ± 1.77 b | 268.65 ± 5.24 a | y = 3.79x−14.12 | 0.9980 | 2.12 | 7.08 |
| 2-Hydroxycinnamic Acid | nd | nd | nd | y = 16.72x−26.94 | 0.9996 | 0.61 | 2.03 |
| 3,4-Dihydroxyphenylacetic Acid | nd | 3.20 ± 0.21 b | 17.87 ± 0.11 a | y = 5.13x−12.39 | 0.9990 | 1.35 | 4.51 |
| 3-Hydroxybenzoic Acid | 10.75 ± 0.38 a | 9.16 ± 0.58 ab | 8.12 ± 0.72 c | y = 3.69x−12.29 | 0.9991 | 1.86 | 6.20 |
| 4-Hydroxybenzoic Acid | 74.92 ± 0.60 b | 76.71 ± 0.88 b | 94.84 ± 1.44 a | y = 7.62x+22.79 | 0.9996 | 1.72 | 5.72 |
| Apigenin | 1457.99 ± 24.48 a | 1270.90 ± 0.30 b | 201.25 ± 3.53 c | y = 11.29x+38.05 | 0.9987 | 0.96 | 3.20 |
| Apigenin 7-glucoside | 293.07 ± 1.22 b | 847.22 ± 5.96 a | nd | y = 21.33x−31.69 | 0.9983 | 0.41 | 1.35 |
| Caffeic acid | 17.42 ± 0.07 c | 44.12 ± 0.92 b | 415.94 ± 2.44 a | y = 11.09x+16.73 | 0.9997 | 3.15 | 10.50 |
| Chlorogenic Acid | 48.33 ± 13.46 c | 136.14 ± 1.56 b | 2135.92 ± 2.02 a | y = 12.14x+32.34 | 0.9995 | 0.55 | 1.82 |
| Eriodictyol | 82.66 ± 0.31 a | 31.32 ± 0.54 b | 7.39 ± 0.25 c | y = 14.24x−0.50 | 0.9998 | 0.80 | 2.68 |
| Ferulic Acid | 1478.13 ± 24.89 b | 1540.91 ± 1.31 a | 1118.92 ± 7.20 c | y = 3.32x−4.30 | 0.9992 | 1.43 | 4.76 |
| Gallic Acid | nd | 4.13 ± 0.10 a | 3.66 ± 0.07 b | y = 4.82x−26.48 | 0.9988 | 1.46 | 4.88 |
| Hesperidin | 63.47 ± 3.16 c | 2389.83 ± 16.55 a | 158.20 ± 0.13 b | y = 5.98x+0.42 | 0.9993 | 1.73 | 5.77 |
| Hyperoside | 117.67 ± 0.07 c | 956.75 ± 3.28 a | 139.13 ± 4.04 b | y = 16.32x−1.26 | 0.9998 | 0.99 | 3.31 |
| Kaempferol | nd | nd | 3.17 ± 0.36 | y = 0.82x−3.06 | 0.9959 | 3.30 | 10.99 |
| Luteolin | 5347.32 ± 54.96 a | 5339.91 ± 72.76 a | 780.53 ± 32.25 b | y = 8.96x+26.80 | 0.9992 | 1.34 | 4.46 |
| Luteolin 7-glucoside | 270.77 ± 3.42 b | 1281.14 ± 39.03 a | 9.80 ± 0.66 c | y = 45.25x+156.48 | 0.9996 | 0.45 | 1.51 |
| p-Coumaric Acid | 97.06 ± 1.72 c | 109.55 ± 0.73 b | 174.73 ± 2.53 a | y = 17.51x+53.73 | 0.9997 | 1.93 | 6.44 |
| Pinoresinol | 262.78 ± 0.40 a | 79.11 ± 0.85 c | 107.54 ± 0.52 b | y = 0.80x−2.69 | 0.9966 | 3.94 | 13.12 |
| Protocatechuic Acid | 74.35 ± 1.88 c | 175.75 ± 1.86 b | 378.73 ± 1.26 a | y = 5.65x−9.99 | 0.9990 | 1.17 | 3.88 |
| Pyrocatechol | nd | nd | nd | y = 0.11x−0.52 | 0.9916 | 9.62 | 32.08 |
| Quercetin | nd | 12.82 ± 0.20 b | 57.12 ± 0.70 a | y = 14.68x−18.25 | 0.9997 | 1.23 | 4.10 |
| Rosmarinic Acid | 29.39 ± 1.49 c | 524.65 ± 14.24 b | 2074.40 ± 3.55 a | y = 9.82x−17.98 | 0.9989 | 0.57 | 1.89 |
| Sinapic Acid | 449.82 ± 15.76 a | 35.79 ± 0.30 b | 23.75 ± 0.77 b | y = 2.09x−6.79 | 0.9974 | 2.64 | 8.78 |
| Syringic Acid | 76.05 ± 3.45 c | 103.67 ± 2.51 b | 157.88 ± 2.09 a | y = 0.74x−1.54 | 0.9975 | 3.75 | 12.50 |
| Taxifolin | nd | nd | 0.95 ± 0.32 | y = 12.32x+9.98 | 0.9993 | 1.82 | 6.05 |
| Vanillic Acid | 694.01 ± 64.38 a | 735.80 ± 24.67 a | 476.23 ± 9.98 b | y = 0.49x−1.61 | 0.9968 | 2.56 | 8.54 |
| Vanillin | 31.36 ± 1.39 a | 15.89 ± 0.29 b | nd | y = 2.02x+135.49 | 0.9926 | 15.23 | 50.77 |
| Verbascoside | 41.47 ± 12.61 a | 4.57 ± 0.31 b | 41.99 ± 0.66 a | y = 8.59x−28.05 | 0.9988 | 0.82 | 2.75 |
x Within each row, means sharing the different superscripts show a comparison between the samples by Tukey’s test at p < 0.05. nd: not detected. LOD and LOQ: limit of detection and limit of quantification, respectively.
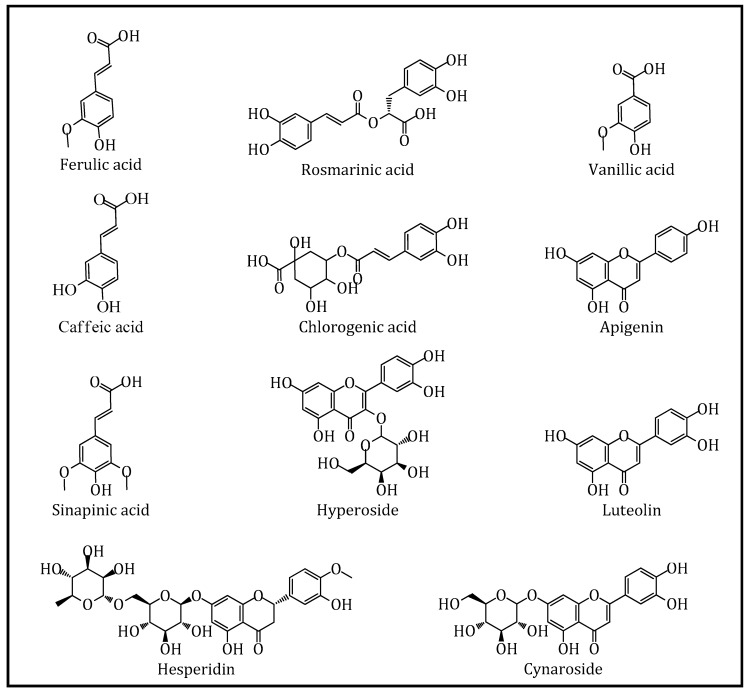
The major phenolic compounds identified in Z. taurica subsp. taurica are shown in Figure 2, while the concentrations (in μg/g extract) and analytical characteristics of selected phytochemicals observed in the Z. taurica subsp. taurica extracts are reported in Table 2.
Figure 2.
Major phenolic compounds identified in the solvent extracts from Z. taurica subsp. taurica.
3.3. Biological Activities
3.3.1. Antioxidant Activities
Some synthetic antioxidant compounds were found to be toxic and carcinogenic in animal models, so they need to be replaced with new and safe antioxidants of natural origin. Antioxidant activity of plant products has often been related to the phenolic content [21]. Possible mechanisms related to the antioxidant activity of these compounds include scavenging of free radicals and absorption of oxygen radicals, etc. [22].
Free radical scavenging activity of DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) radical cation scavenging activity, the phosphomolybdenum assay, the cupric ion reducing (CUPRAC) method, the ferric reducing antioxidant power (FRAP) method, and metal chelating activity on ferrous ions were employed to evaluate the antioxidant capacity of Z. taurica subsp. taurica extracts.
Results obtained for all different extracts are reported in Table 3. As it was observed that the properties of the extracting solvents significantly affected total phenolic content and antioxidant capacity [21], comparing the antioxidant activity of the different extracts will enable us to establish a standardized procedure for sample preparation. All assays showed that methanol and aqueous extracts showed the highest antioxidant capacity. This observed antioxidant activity could be due to the greater presence of secondary bioactive metabolites belonging to the flavonoids noticed in the methanol extract.
Table 3.
Antioxidant activities of standards and the extracts from Z. taurica subsp. taurica x.
| Assays | ZTT-EtOAc | ZTT-MeOH | ZTT-W | Trolox | EDTA |
|---|---|---|---|---|---|
| Inhibition Concentration (IC50: mg/mL) | |||||
| DPPH Radical Scavenging | 15.75 ± 0.66 c | 5.74 ± 0.08 b | 7.02 ± 0.23 b | 0.25 ± 0.01 a | - |
| ABTS Radical Scavenging | 6.30 ± 0.03 d | 2.74 ± 0.10 c | 2.39 ± 0.10 b | 0.26 ± 0.01 a | - |
| Phosphomolybdenum | 2.60 ± 0.03 c | 1.84 ± 0.08 b | 3.80 ± 0.17 d | 1.15 ± 0.01 a | - |
| CUPRAC Reducing Power | 2.39 ± 0.10 b | 2.24 ± 0.11 b | 2.80 ± 0.02 c | 0.31 ± 0.02 a | - |
| FRAP Reducing Power | 3.41 ± 0.28 c | 1.42 ± 0.04 b | 1.71 ± 0.02 b | 0.1 ± 0.01 a | - |
| Ferrous Ion Chelating | 51.40 ± 1.32 c | 3.77 ± 0.09 b | 1.04 ± 0.01 b | - | 0.034 ± 0.003 a |
| Antioxidant Activity | |||||
| DPPH Radical Scavenging (mg TE/g extract) | 15.01 ± 0.68 c | 42.15 ± 0.6 1a | 33.99 ± 1.21 b | - | - |
| ABTS Radical Scavenging (mg TE/g extract) | 42.06 ± 0.22 c | 97.01 ± 3.45 b | 111.94 ± 4.74 a | - | - |
| Phosphomolybdenum (mg TE/g extract) | 446.22 ± 4.94 b | 630.08 ± 28.80 a | 304.30 ± 13.99 c | - | - |
| CUPRAC Reducing Power (mg TE/g extract) | 131.89 ± 5.95 a | 134.66 ± 7.00 a | 105.47 ± 0.70 b | - | - |
| FRAP Reducing Power (mg TE/g extract) | 30.79 ± 2.55 c | 73.62 ± 1.85 a | 61.27 ± 0.67 b | - | - |
| Ferrous Ion Chelating (mg EDTAE/g extract) | 1.03 ± 0.04 c | 18.53 ± 0.47 b | 69.31 ± 0.18 a | - | - |
x Within each row, means sharing the different superscripts show a comparison between the samples by Tukey’s test at p<0.05. TE and EDTA: Trolox and Ethylenediaminetetraacetic acid (disodium salt) equivalents, respectively.
3.3.2. Enzyme Inhibitory Activities
Several papers have already shown the beneficial activity of phenolic compounds on tyrosinase and α-amylase inhibition [2,14,23].
All the extracts of Z. taurica subsp. taurica inhibited the studied enzymes (Table 4). ZTT-EtOAc extract presented the highest inhibition activity against tyrosinase (1.37 mg/mL) followed by ZTT-MeOH extract (1.46 mg/mL), with the ZTT-W extract exhibiting the lowest inhibition activity (2.29 mg/mL). Kojic acid was used as a reference. Acarbose, which is considered a strong inhibitor against α-amylase, was used as a reference in this enzyme inhibitory assay. Against α-amylase, ZTT-EtOAc extract presented the highest inhibition activity (1.82 mg/mL) followed by ZTT-MeOH extract (2.69 mg/mL). ZTT-W extract again demonstrated the lowest inhibition activity (62.56 mg/mL). Acarbose was used as a reference.
Table 4.
Enzyme inhibition activities of standards and the solvent extracts from Z. taurica subsp. taurica x.
| Assays | ZTT-EtOAc | ZTT-MeOH | ZTT-W | Kojic acid | Acarbose |
|---|---|---|---|---|---|
| Inhibition Concentration (IC50: mg/mL) | |||||
| Tyrosinase Inhibition | 1.37 ± 0.07 b | 1.46 ± 0.06 b | 2.29 ± 0.13 c | 0.37 ± 0.02 a | - |
| α-Amylase Inhibition | 1.82 ± 0.08 ab | 2.69 ± 0.14 b | 62.56 ± 0.56 c | - | 1.21 ± 0.07 a |
| Enzyme inhibition activity | |||||
| Tyrosinase Inhibition (mg KAE/g extract) | 262.76 ± 13.82 a | 246.27 ± 9.75 a | 156.88 ± 8.88 b | - | - |
| α-Amylase Inhibition (mg ACE/g extract) | 672.87 ± 28.68 a | 452.44 ± 23.81 b | 16.03 ± 0.18 c | - | - |
x Within each row, means sharing the different subscripts show a comparison between the samples by Tukey’s test at p < 0.05. KAE and ACE: kojic acid and acarbose equivalent.
These results, in accordance with previously work [10], suggest that the phytochemicals responsible for tyrosinase and α-amylase inhibition could have low polarity (non-polar extracts showed higher activity).
3.4. Correlations among Phenolic Compounds and Assays
The correlation between phenolic compounds and biological assays (antioxidant and enzyme inhibitor assays) is reported in Table 5, where the Pearson correlation coefficients are shown.
Table 5.
Correlations among phenolic compounds and assays x.
| Assays and Compounds | Tyrosinase | α-Amylase | CUPRAC | FRAP | ABTS | DPPH | Phosphomolybdenum | Ferrous Ion Chelating |
|---|---|---|---|---|---|---|---|---|
| α-Amylase | 0.982 | 1 | ||||||
| CUPRAC | 0.973 | 0.912 | 1 | |||||
| FRAP | –0.376 | –0.544 | –0.153 | 1 | ||||
| ABTS | –0.766 | –0.874 | –0.599 | 0.883 | 1 | |||
| DPPH | –0.363 | –0.533 | –0.14 | 0.999 z | 0.877 | 1 | ||
| Phosphomolybdenum | 0.736 | 0.595 | 0.872 | 0.350 | –0.13 | 0.363 | 1 | |
| Ferrous Ion Chelating | –0.995 | –0.996 | –0.944 | 0.470 | 0.829 | 0.458 | –0.662 | 1 |
| Total Flavonoid | 0.751 | 0.612 | 0.883 | 0.330 | –0.152 | 0.342 | 0.999 y | –0.679 |
| Total Phenolic | 0.973 | 0.999 y | 0.895 | –0.578 | –0.893 | –0.567 | 0.562 | –0.992 |
| Caffeic Acid | –0.996 | –0.962 | –0.989 | 0.295 | 0.709 | 0.282 | –0.791 | 0.982 |
| Vanillic Acid | 0.957 | 0.884 | 0.988 | –0.09 | –0.543 | –0.091 | 0.901 | –0.919 |
| Chlorogenic Acid | –0.994 | –0.956 | –0.992 | 0.273 | 0.693 | 0.260 | –0.805 | 0.978 |
| Sinapic Acid | 0.639 | 0.774 | 0.446 | –0.953 | –0.984 | –0.949 | –0.049 | –0.716 |
| Ferulic Acid | 0.960 | 0.890 | 0.999 y | –0.101 | –0.556 | –0.088 | 0.896 | –0.926 |
| Luteolin 7-glucoside | 0.543 | 0.374 | 0.721 | 0.574 | 0.124 | 0.585 | 0.968 | –0.453 |
| Hesperidin | 0.336 | 0.151 | 0.543 | 0.747 | 0.348 | 0.756 | 0.884 | –0.236 |
| Hyperoside | 0.348 | 0.164 | 0.554 | 0.738 | 0.335 | 0.747 | 0.891 | –0.249 |
| Rosmarinic Acid | –0.996 | –0.995 | –0.949 | 0.456 | 0.820 | 0.444 | –0.673 | 0.999 z |
| Luteolin | 0.990 | 0.945 | 0.996 | –0.239 | –0.666 | –0.226 | 0.826 | –0.969 |
| Apigenin | 0.999 z | 0.981 | 0.975 | –0.369 | –0.762 | –0.357 | 0.741 | –0.994 |
x Data show the Pearson correlation coefficients between the parameters. y Significant at p < 0.05; z Significant at p < 0.01.
Correlations between total phenolic and flavonoid compounds and tyrosinase assay were 0.97 and 0.75, respectively. Similarly, the correlation between total phenolic and flavonoid compounds, and α-amylase assay were 0.99 and 0.61, respectively. Correlations between total phenolic compounds and antioxidant capacity methods CUPRAC and phosphomolybdenum were 0.89 and 0.56, respectively. Correlations between total flavonoid content and antioxidant capacity methods CUPRAC, FRAP, DPPH, and phosphomolybdenum were 0.88, 0.33, 0.34, and 0.99, respectively.
4. Conclusions
The present study examined the phenolic composition, the antioxidative activity, and the enzyme inhibitory activity against α-amylase and tyrosinase from Z. taurica subsp. taurica extracts using three different solvents. All extracts showed high phenolic content. We showed a significant antioxidant capacity and an inhibitory capacity against α-amylase and tyrosinase enzymes. Ziziphora taurica subsp. taurica can be considered a powerful natural antioxidant. To date, some of the studies concerning Ziziphora have been conducted in vitro, so in vivo investigations of this species are necessary in future studies.
Supplementary Materials
All analytical methods applied for phenolic composition (TPC, TFC), antioxidant and enzyme inhibitory activities were given in the supplementary materials (S1). The supplementary materials are available online at https://www.mdpi.com/2218-273X/9/8/367/s1.
Author Contributions
Data curation: A.T. and V.F.; investigation: C.S. and O.C.; methodology: M.L., M.T., and C.S.; project administration: C.S.; supervision: M.L., M.T., and C.S.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Shahbazi Y. Chemical compositions, antioxidant and antimicrobial properties of Ziziphora clinopodioides Lam. essential oils collected from different parts of Iran. J. Food Sci. Technol. 2017;54:3491–3503. doi: 10.1007/s13197-017-2806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mocan A., Diuzheva A., Badarau S., Moldovan C., Andruch V., Carradori S., Campestre C., Tartaglia A., De Simone M., Vodnar D., et al. Liquid phase and microwave-assisted extractions for multicomponent phenolic pattern determination of five Romanian Galium species coupled with bioassays. Molecules. 2019;24:1226. doi: 10.3390/molecules24071226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekut M., Brkic S., Kladar N., Dragovic G., Gavaric N., Bozin B. Potential of selected Lamiaceae plants in anti(retro)viral therapy. Pharmacol. Res. 2018;133:301–314. doi: 10.1016/j.phrs.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Šmejkal K., Malaník M., Zhaparkulova K., Sakipova Z., Ibragimova L., Ibadullaeva G., Žemlicka M. Kazakh Ziziphora species as sources of bioactive substances. Molecules. 2016;21:826. doi: 10.3390/molecules21070826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammadhosseini M. The ethnobotanical, phytochemical and pharmacological properties and medicinal applications of essential oils and extracts of different Ziziphora species. Ind. Crop. Prod. 2017;105:164–192. doi: 10.1016/j.indcrop.2017.05.009. [DOI] [Google Scholar]
- 6.Ozturk S., Ercisli S. Antibacterial activity and chemical constitutions of Ziziphora clinopodioides. Food Control. 2007;18:535–540. doi: 10.1016/j.foodcont.2006.01.002. [DOI] [Google Scholar]
- 7.Senejoux F., Demougeot C., Kerram P., Aisa H.A., Berthelot A., Bevalot F., Girard-Thernier C. Bioassay-guided isolation of vasorelaxant compounds from Ziziphora clinopodioides Lam. (Lamiaceae) Fitoterapia. 2012;83:377–382. doi: 10.1016/j.fitote.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Benayad Z., Martinez-Villaluenga C., Frias J., Gomez-Cordoves C., Es-Safi N.E. Phenolic composition, antioxidant and anti-inflammatory activities of extracts from Moroccan Opuntia ficus-indica flowers obtained by different extraction methods. Ind. Crops Prod. 2014;62:412–420. doi: 10.1016/j.indcrop.2014.08.046. [DOI] [Google Scholar]
- 9.Wang H.M., Chen C.Y., Chen C.Y., Ho M.L., Chou Y.T., Chang H.C., Lee C.H., Wang C.Z., Chu I.M. (-)-N-Formylanonaine from Michelia alba as a human tyrosinase inhibitor and antioxidant. Bioorg. Med. Chem. 2010;18:5241–5247. doi: 10.1016/j.bmc.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 10.Sarikurkcu C., Kirkan B., Ozer M.S., Ceylan O., Atilgan N., Cengiz M., Tepe B. Chemical characterization and biological activity of Onosma gigantea extracts. Ind. Crop. Prod. 2018;115:323–329. doi: 10.1016/j.indcrop.2018.02.040. [DOI] [Google Scholar]
- 11.Genovese S., Fiorito S., Locatelli M., Carlucci G., Epifano F. Analysis of biologically active oxyprenylated ferulic acid derivatives in Citrus fruits. Plant Food Hum. Nutr. 2014;69:255–260. doi: 10.1007/s11130-014-0427-8. [DOI] [PubMed] [Google Scholar]
- 12.Zengin G., Llorent-Martínez E.J., Córdova M.L.F.-D., Bahadori M.B., Mocan A., Locatelli M., Aktumsek A. Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca. Ind. Crop. Prod. 2018;111:11–21. doi: 10.1016/j.indcrop.2017.09.065. [DOI] [Google Scholar]
- 13.Sarikurkcu C., Tepe B., Kocak M.S., Uren M.C. Metal concentration and antioxidant activity of edible mushrooms from Turkey. Food Chem. 2015;175:549–555. doi: 10.1016/j.foodchem.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Tlili N., Kirkan B., Sarikurkcu C. LC–ESI–MS/MS characterization, antioxidant power and inhibitory effects on α-amylase and tyrosinase of bioactive compounds from hulls of Amygdalus communis: The influence of the extracting solvents. Ind. Crop. Prod. 2019;128:147–152. doi: 10.1016/j.indcrop.2018.11.014. [DOI] [Google Scholar]
- 15.Cittan M., Çelik A. Development and validation of an analytical methodology based on liquid chromatography–electrospray tandem mass spectrometry for the simultaneous determination of phenolic compounds in olive leaf extract. J. Chromatogr. Sci. 2018;56:336–343. doi: 10.1093/chromsci/bmy003. [DOI] [PubMed] [Google Scholar]
- 16.Apak R., Güçlü K., Özyürek M., Esin Karademir S., Erçaǧ E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006;57:292–304. doi: 10.1080/09637480600798132. [DOI] [PubMed] [Google Scholar]
- 17.Kocak M.S., Sarikurkcu C., Cengiz M., Kocak S., Uren M.C., Tepe B. Salvia cadmica: Phenolic composition and biological activity. Ind. Crop. Prod. 2016;85:204–212. doi: 10.1016/j.indcrop.2016.03.015. [DOI] [Google Scholar]
- 18.Odabas Kose E., Aktaş O., Deniz I.G., Sarikürkçü C. Chemical composition, antimicrobial and antioxidant activity of essential oil of endemic Ferula lycia Boiss. J. Med. Plants Res. 2010;4:1698–1703. [Google Scholar]
- 19.Zengin G., Sarikurkcu C., Uyar P., Aktumsek A., Uysal S., Kocak M.S., Ceylan R. Crepis foetida L. subsp rhoeadifolia (Bleb.) Celak. as a source of multifunctional agents: Cytotoxic and phytochemical evaluation. J. Funct. Foods. 2015;17:698–708. doi: 10.1016/j.jff.2015.06.041. [DOI] [Google Scholar]
- 20.Kuo C.T., Liu T.H., Hsu T.H., Lin F.Y., Chen H.Y. Antioxidant and antiglycation properties of different solvent extracts from Chinese olive (Canarium album L.) fruit. Asian. Pac. J. Trop. Med. 2015;8:1013–1021. doi: 10.1016/j.apjtm.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Michiels J.A., Kevers C., Pincemail J., Defraigne J.O., Dommes J. Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 2012;130:986–993. doi: 10.1016/j.foodchem.2011.07.117. [DOI] [Google Scholar]
- 22.Deng Y., Yang G., Yue J., Qian B., Liu Z., Wang D., Zhong Y., Zhao Y. Influences of ripening stages and extracting solvents on the polyphenolic compounds, antimicrobial and antioxidant activities of blueberry leaf extracts. Food Control. 2014;38:184–191. doi: 10.1016/j.foodcont.2013.10.023. [DOI] [Google Scholar]
- 23.Zengin G., Sarikurkcu C., Gunes E., Uysal A., Ceylan R., Uysal S., Gungord H., Aktumsek A. Two Ganoderma species: Profiling of phenolic compounds by HPLC–DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer’s disease and skin disorders. Food Funct. 2015;6:2794–2802. doi: 10.1039/C5FO00665A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.