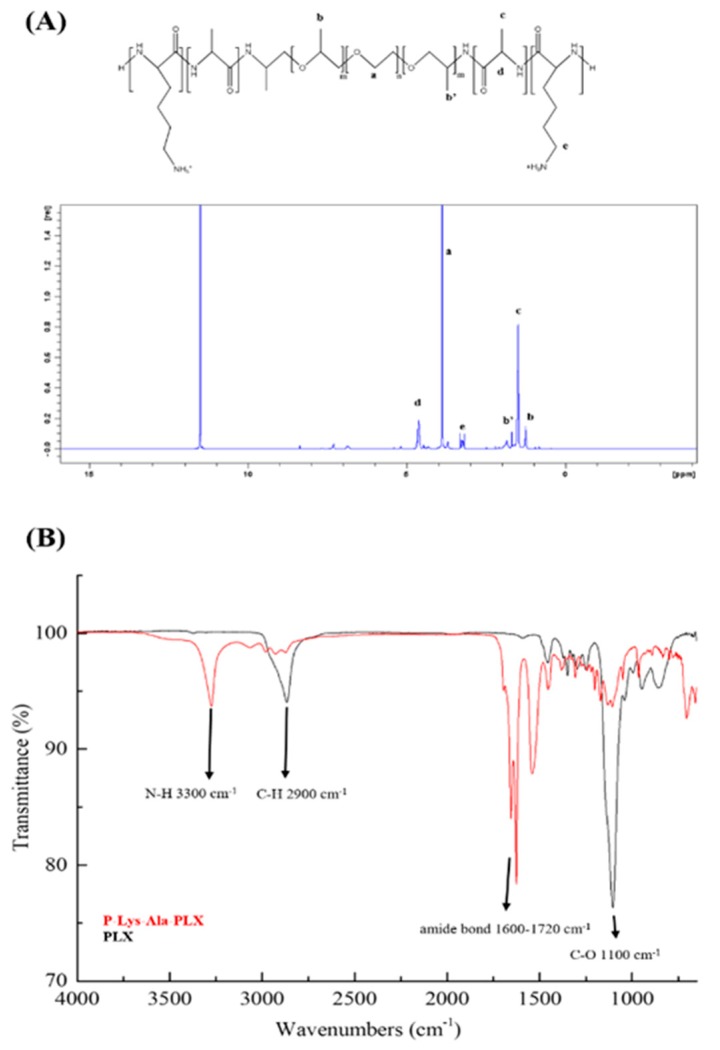
Figure 1.
(A) 1H-NMR of poloxamer (PLX)-poly(l-alanine-lysine) (P–Lys–Ala–PLX) copolymer in trifluoroacetic acid-d (TFA-d). (B) FT-IR spectra of P–Lys–Ala–PLX (red line) and PLX (black line) samples obtained via an attenuated total reflectance (ATR) module (2900 cm−1, alkane of Pluronic F-127; 3345 cm−1, amide of poly(l-alanine) and poly(l-lysine); 1637 cm−1 and 1540 cm−1, amide I and II of poly(l-alanine) and poly(l-lysine); 1100 cm−1, carbon-oxygen bond of PLX.