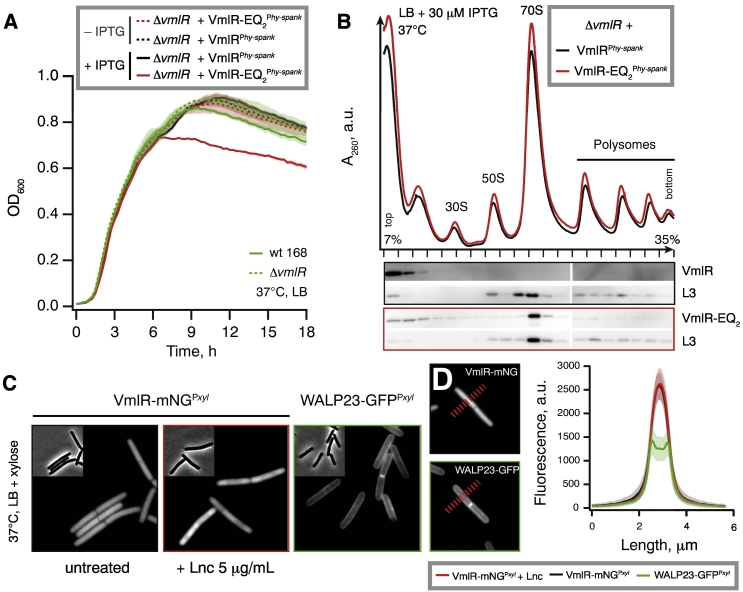
Fig. 4.
B. subtilis ARE VmlR is a cytoplasmic protein that directly protects the ribosome from antibiotics. (A) Growth of wild-type B. subtilis 168, isogenic ΔvmlR knockout as well as ΔvmlR knockout expressing either wild-type or EQ2 version of VmlR under the control of IPTG-inducible Phy-spank promoter. Six biological replicates were averaged for each growth curve and the data presented as geometric means ± standard deviation. (B) Polysome analysis and Western blotting of ΔvmlR B. subtilis expressing C-terminally HTF-tagged wild-type and EQ2 version of VmlR. (C) Phase contrast and fluorescence images of uninhibited B. subtilis cells expressing VmlR-mNeonGreen (VmlR-mNG) in the presence and absence of lincomycin (40-min incubation with 5 μg/mL) and a model transmembrane protein WALP23-GFP are shown for comparison. (D) Fluorescence intensity profiles were measured perpendicular to the cell length axis along a 325-nm-wide and 5.8-μm-long line as indicated. Fluorescence intensity profiles of cells expressing WALP23-GFP [62] and cells expressing VmlR-mNG in the presence and absence of lincomycin. The graph depicts the average fluorescence intensity profiles and the corresponding standard deviations (n = 30).