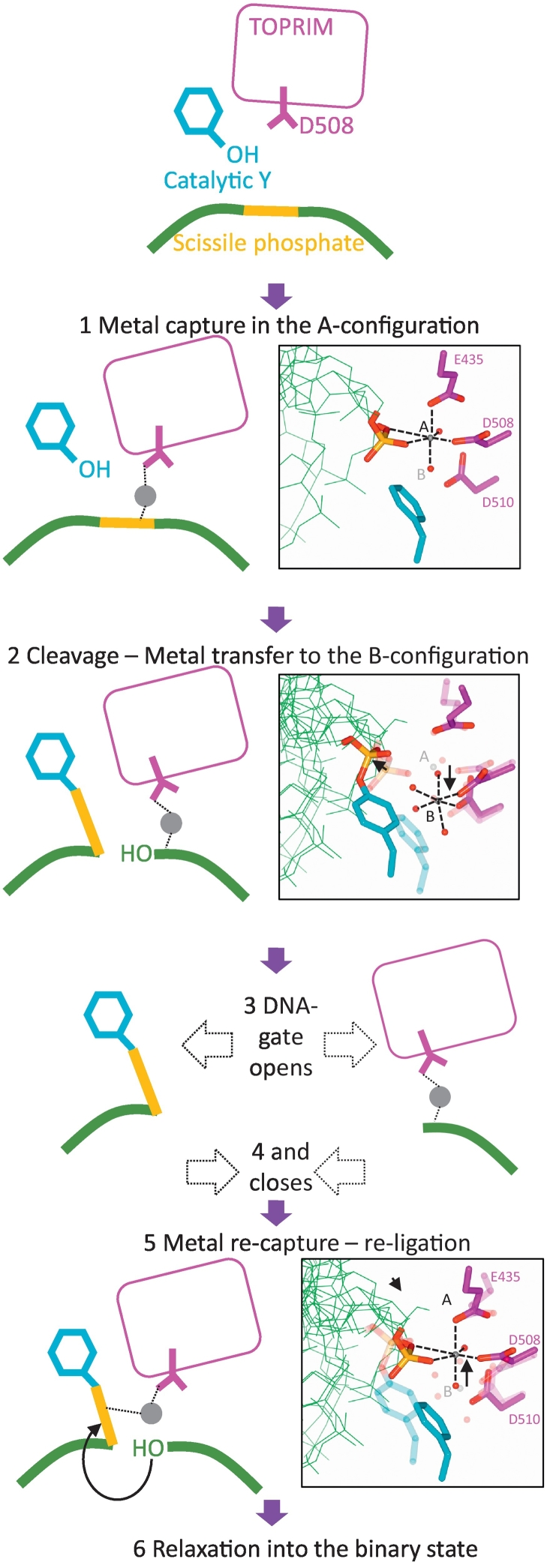
Fig. 7.
Dynamic model for the cleavage–religation cycle catalyzed by a single metal ion. (1) Relative movement of the GyrA subunit (see Fig. 3), extension of the DNA and tilting of the TOPRIM domain create a coordination environment for a single metal ion, the A-configuration, involving Asp508 residue from the TOPRIM domain, water molecules, and two oxygens from the scissile phosphate (see inset). (2) Through its contacts with the scissile phosphate, the metal catalyzes phosphotransfer to the catalytic tyrosine. The phospho-tyrosine then moves away after cleavage, disrupting the A-configuration, which favors the transfer of the metal to the B-configuration (inset) initially filled by a water molecule contacting the A-configuration metal. (3) Opening of the DNA gate obliterates the A-configuration but does not affect the B-configuration, allowing the metal ion to be stored in the B-configuration during strand passage. Keeping the catalytic metal away from the phospho-tyrosine can prevent detrimental hydrolysis of the bond during strand passage. (4) Closure of the DNA gate brings the phospho-tyrosine closer to the catalytic metal in the B-configuration. (5) We hypothesize that phospho-tyrosine is being brought even closer to the B-configuration coordination cage and can disrupt a coordinating water molecule, resulting in metal recapture by the phosphate and the catalysis of phosphotransfer to the 3´-OH (which remains close to the B-configuration during the cycle). This re-creates the A-configuration and panel 6. Relaxation into the binary state can then ensure irreversibility by disrupting the A-configuration. We envision that controlling the motion of the DNA and the position of the scissile phosphate allows the enzyme to control cleavage and religation. This control is presumably coupled to the ATP hydrolysis and exchange cycle. Alternatively, a compound binding the enzyme might also influence the position of the scissile phosphate.