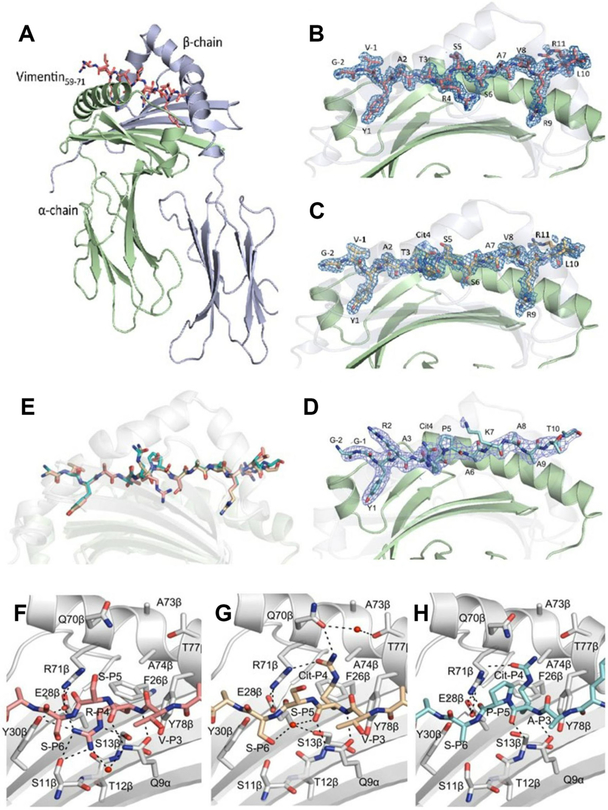
Figure 3.
Crystal structure of HLA-DRB1*1402 in complex with vimentin59–71, vimentin-64Cit59–71 and fibrinogen β 74Cit69–81. The α-chains and β-chains of (A) the HLA-DRB1*1402 in complex with vimentin59–71 are shown in cartoon representation and coloured in light green and light blue, respectively, while peptide is displayed as sticks. Side view of (B) HLA-DRB1*1402-vimentin59–71, (C) HLA-DRB1*1402-vimentin-64Cit59–71 and (D) HLA-DRB1*1402-fibrinogen β74Cit69–81. The carbons are coloured deep salmon, light orange and teal for vimentin59–71, vimentin-64Cit59–71 and fibrinogen β74Cit69–81, respectively; nitrogens are coloured in blue and oxygens are coloured in red. The β-chain is transparent to help visualise the peptide. The unbiased 2Fo-Fc electron density map of the peptides is shown in blue and contoured to 1 σ. (E) Superposition of the three peptides bound to HLA-DRB1*1402. The P4 pocket of HLA-DRB1*1402 bound to (F) vimentin59–71, (G) vimentin-64Cit59–71 and (H) fibrinogenβ74Cit69–81. The P4-Arg in the vimentin59–71 peptide is buried in the P4 pocket. The P4-Cit of the vimentin-64Cit59–71 and fibrinogenβ74Cit69–81 peptide adopts an upright conformation.