Figure 7.
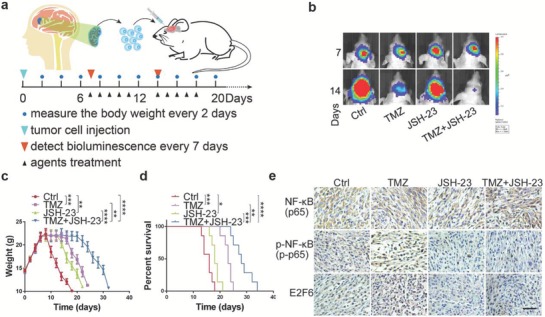
JSH‐23 treatment enhances the therapeutic efficacy of TMZ. a) Schematic illustration of the evaluation of the therapeutic efficacy of TMZ or JSH23 alone and their combination in vivo. Mouse GBM models were established by transplanting primary GBM cells isolated from fresh GBM samples into mouse brain. Mice bearing GBM were intraperitoneally treated with TMZ (5 mg kg−1 d−1), JSH‐23 (6 mg kg−1 d−1), TMZ (5 mg kg−1 d−1)/JSH‐23 (6 mg kg−1 d−1), or PBS vehicle control for 2 weeks (5 days on and 2 days off). Tumor growth was evaluated by bioluminescence once a week, and the mouse body weights were measured every 2 days. b) Representative bioluminescence images of the intracranial PDX mice treated with indicated agents at days 7 and 14 (mean ± SD, n = 7, **P < 0.01, ***P < 0.001, ****P < 0.0001). c) Body weights of PDX mice treated with indicated agents (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). d) Kaplan–Meier curve shows that the overall survival time was increased in TMZ/JSH‐23 combination treatment group (**P < 0.01, ***P < 0.001, ****P < 0.0001). e) Representative immunostaining results of expression of NF‐κB, p‐NF‐κB, and E2F6 in PDX tumors after treatment with indicated agents. Scale bar: 50 µm.
