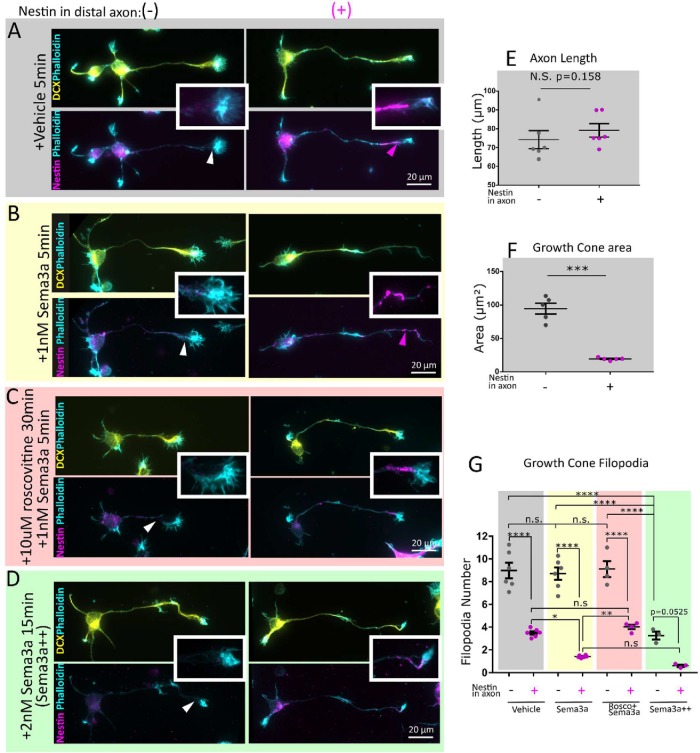
FIGURE 4:
Nestin-expressing neurons have smaller growth cones with fewer filopodia and are more sensitive to Sema3a in a cdk5-dependent manner. E16 mouse cortical neurons 1DIV (stage 3) were immunostained against DCX (to confirm neuronal identity), phalloidin (to visualize the growth cones), and nestin. Cells were characterized based on nestin immunoreactivity in the distal axon nestin as Nes(–)—not detected, Nes(+)—nestin-positive. Conditions are untreated (A), 1 nM Sema3a 5 min (B), pretreated with 10 µM Roscovitine 30 min prior to 1 nM Sema3a treatment for 5 min (C), and a Sema3a++ treatment of 2 nM Sema3a for 15 min (D). Insets allow appreciation of the filamentous nature of the nestin immunostaining and proximity to the growth cone. Channel intensity is adjusted equally for nestin and DCX. At low magnification, phalloidin labeling has been adjusted variably to show morphology, but in the insets the phalloidin channel was adjusted in an equal manner. Nestin-expressing neurons are not significantly different by axon length (E), but have significantly smaller axon growth cones (F). Neuron morphology was quantified (30–40 cells per independent experiment). Mean of each experiment and SEM are indicated. Statistical test was a t test with n = 6 (E) or n = 5 (F). (G) Neurons with nestin have fewer filopodia than those without nestin. In cells treated with 1 nM Sema3a for 5 min, the nestin-positive cells had a significant reduction in filopodia in comparison with untreated nestin-positive cells, whereas the nestin-negative cells did not. Conditions have been color coded for ease of comparison. Roscovitine inhibited the decrease in filopodia number after Sema3a treatment. When a high dose (2 nM) and longer treatment (15 min) of Sema3a are used, both nestin-negative and -positive neurons retract filopodia and significantly so, indicating that nestin is not absolutely required for Sema3a-induced filopodial retraction, but increases Sema3a sensitivity. Filopodia number was quantified for 6 (vehicle, Sema3a), 4 (roscovitine + Sema3a), or 3 (Sema3a++) independent experiments. An average of 30–40 cells quantified per experiment is shown. Mean of each experiment and SEM are indicated. Normality was confirmed with the Shapiro–Wilk normality test. Statistical test was one-way ANOVA with Tukey’s multiple comparison correction, with n = number of experiments. Each condition was compared with every other condition.