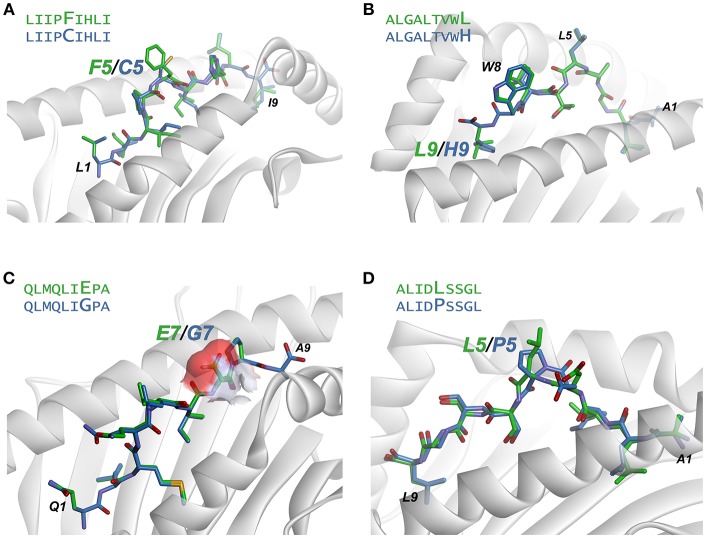
Figure 6.
Examination of modeled structures of select neoantigens and their wild-type counterparts. (A) The neoantigen LIIPFIHLI substitutes a phenylalanine for a cysteine at position 5. The position 5 side chain is predicted to extend from the top of a bulge in the peptide, and the mutation results in an increase in exposed hydrophobic surface of 90 Å2. (B) The neoantigen ALGALTVWL substitutes a leucine for a histidine at position 9, “fixing” the second primary anchor residue and improving peptide binding to HLA-A2. The leucine at position 5 and tryptophan at position 8 are predicted to extend up from the peptide backbone to form interactions with T cell receptors. (C) The neoantigen QLMQLIEPA substitutes a glutamate for a glycine at position 7. The glutamate 7 side chain in the mutant peptide is predicted to be fully exposed, increasing exposed charged surface area as indicated by the surface area representation for the position 7 side chain. (D) The neoantigen ALIDLSSGL replaces a proline with a leucine at position 5 of the peptide. No conformational consequences are predicted for the mutation.