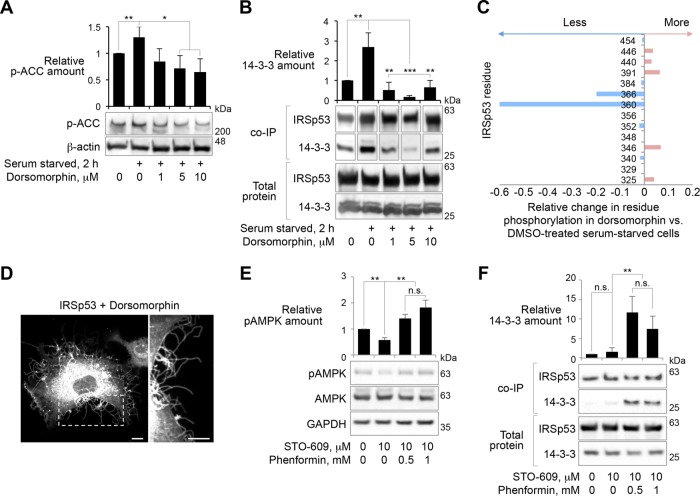
FIGURE 3:
AMPK-mediated phosphorylation controls the binding of 14-3-3 to IRSp53. (A) Effect of serum starvation and dorsomorphin-dependent inhibition of AMPK-related kinases on the phosphorylation of the canonical AMPK substrate acetyl-CoA carboxylase (ACC) in HEK293T cells. (B) Amount of 14-3-3 that coimmunoprecipitates with IRSp53-FLAG from HEK293T cells. Note that neither serum starvation nor dorsomorphin treatment affects the expression of endogenous 14-3-3, whereas starvation increases and dorsomorphin decreases the amount of 14-3-3 that coimmunoprecipitates with IRSp53-FLAG. Abundance is reported relative to fed cells expressing IRSp53-FLAG. (C) Relative change in the fraction of phosphorylated IRSp53 peptides detected by tandem MS/MS in DMSO-treated vs. 5 μM dorsomorphin-treated starved cells. (D) COS-7 cell expressing IRSp53-GFP treated with 5 μM dorsomorphin. Scale bars represent 10 and 5 μm in whole-cell and inset images, respectively. (E) Effect of phenformin treatment on the phosphorylation of residue T172 of the catalytic subunit of AMPK (kinase activation) in HEK293T cells. Cells were pretreated with the Ca2+-CAMKK inhibitor STO-609 to suppress basal phosphorylation of AMPK before treatment with the indicated concentrations of phenformin. (F) Amount of 14-3-3 that coimmunoprecipitates with IRSp53-FLAG from HEK293T cells treated with phenformin. Note that neither STO-609 nor phenformin treatment affect the expression of IRSp53-FLAG or endogenous 14-3-3, whereas phenformin dramatically increases the amount of 14-3-3 that coimmunoprecipitates with IRSp53-FLAG. Abundance is reported relative to untreated fed cells expressing IRSp53-FLAG. Throughout this figure, error bars are ± SD from three independent experiments. The statistical significance of all the measurements was determined using an unpaired t test based on three independent experiments (n.s., nonsignificant; *p < 0.05, **p < 0.01, ***p < 0.001).