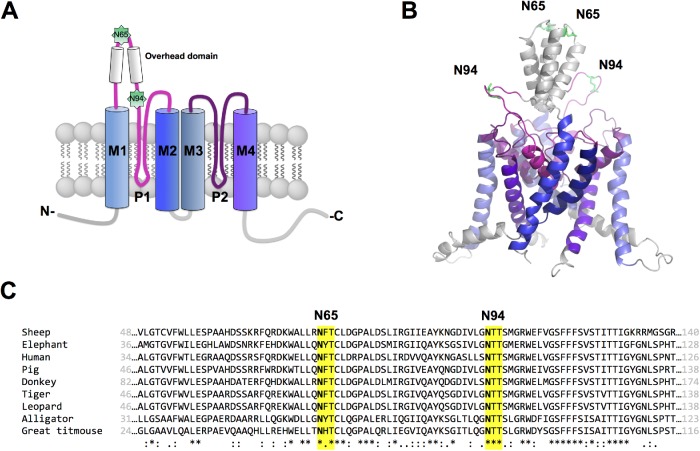
FIGURE 1:
Putative N-glycosylation sites of hK2P17.1. (A) Schematic two-dimensional membrane model of an hK2P17.1 subunit. Putative N-glycosylated asparagine residues 65 and 94 in the M1-P1 linker are highlighted. P, pore-forming domain; M, transmembrane domain; N, N-terminus; C, C-terminus; extracellular site top, intracellular site bottom. (B) Three-dimensional homology model of hK2P17.1, assembled as dimer illustrates that asparagine residues 65 and 94 are directed toward the extracellular site and therefore accessible to N-glycosylation. (C) Species conservation of N-glycosylation motives at asparagine residues 65 and 94. *, full conservation; :, conservative substitution; ., semi-conservative substitution.