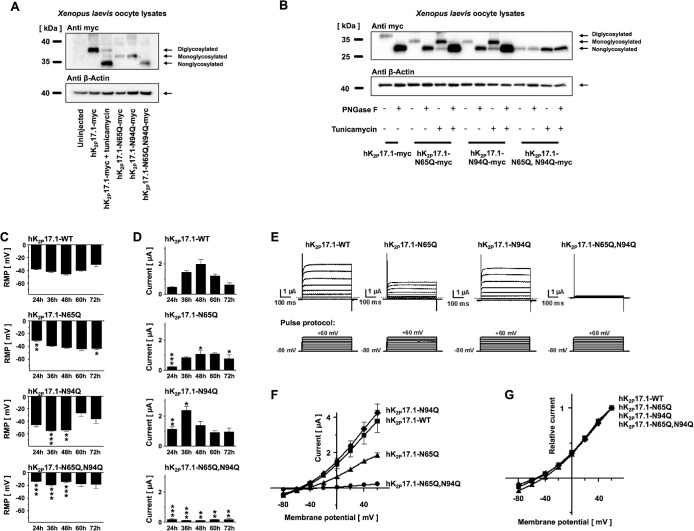
FIGURE 3:
Verification of hK2P17.1 glycosylation sites. (A) Lysates of Xenopus oocytes, expressing the indicated glutamine mutants of hK2P17.1-myc, were separated by SDS–PAGE followed by anti-myc immunoblotting. Elimination of N-glycosylation motives resulted in increased protein mobility. Tunicamycin was administered as indicated. β-Actin immunoreactivity served as loading control. (B) Glutamine mutants of hK2P17.1-myc, heterologously expressed in Xenopus oocytes, were treated with the N-glycosidase PNGase F or the N-glycosylation inhibitor tunicamycin as indicated, followed by SDS–PAGE and anti-myc immunoblotting. β-Actin signals served as loading control. (C, D) Xenopus oocytes were injected with cRNA of either WT hK2P17.1 or indicated glutamine mutants. Measurements were taken at different time points between 24 and 72 h. (C) Resting membrane potential (RMP) of the cells. (D) Outward potassium currents, measured at the end of a 500-ms +20-mV test pulse (n = 4–10). (E) Representative sets of macroscopic potassium current recordings in Xenopus oocytes expressing hK2P17.1-WT or glutamine mutants. Currents were elicited by application of the test pulse protocol as depicted at the bottom. Dotted lines indicate zero current levels. (F, G) Corresponding mean step current amplitudes are plotted as functions of test pulse potentials to compare mean current–voltage relationships of artificially di-, mono-, and nonglycosylated hK2P17.1 monomers. (F) Original current amplitudes. (G) Currents normalized to maximum currents at +60 mV (n = 6–9). Data are given as mean values ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001 for Bonferroni-corrected two-tailed Student’s t tests.