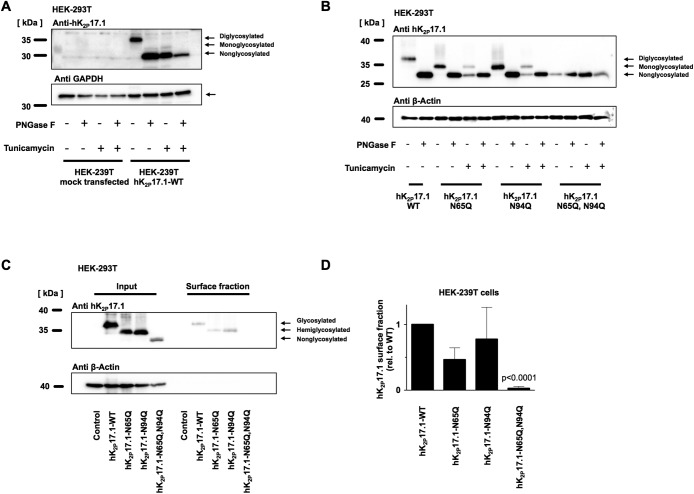
FIGURE 5:
N-glycosylation of hK2P17.1 expressed in mammalian cells. (A) hK2P17.1-WT channel subunits were heterologously expressed in HEK-293T cells in the presence or absence of the N-glycosylation inhibitor tunicamycin. Cell lysates were digested with PNGase F to remove N-linked sugar moieties as indicated. Immunoblots for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as loading control. (B) WT hK2P17.1 channel subunits and glutamine mutants were expressed in HEK-293T cells and treated as described in A. (C) Surface fractions of HEK-293T cells expressing indicated hK2P17.1 variants were isolated by surface biotinylation followed by streptavidin precipitation. (D) Mean optical densities of the surface blots were normalized to the signal of WT hK2P17.1. Data are provided as mean values ± SEM of three independent experiments.