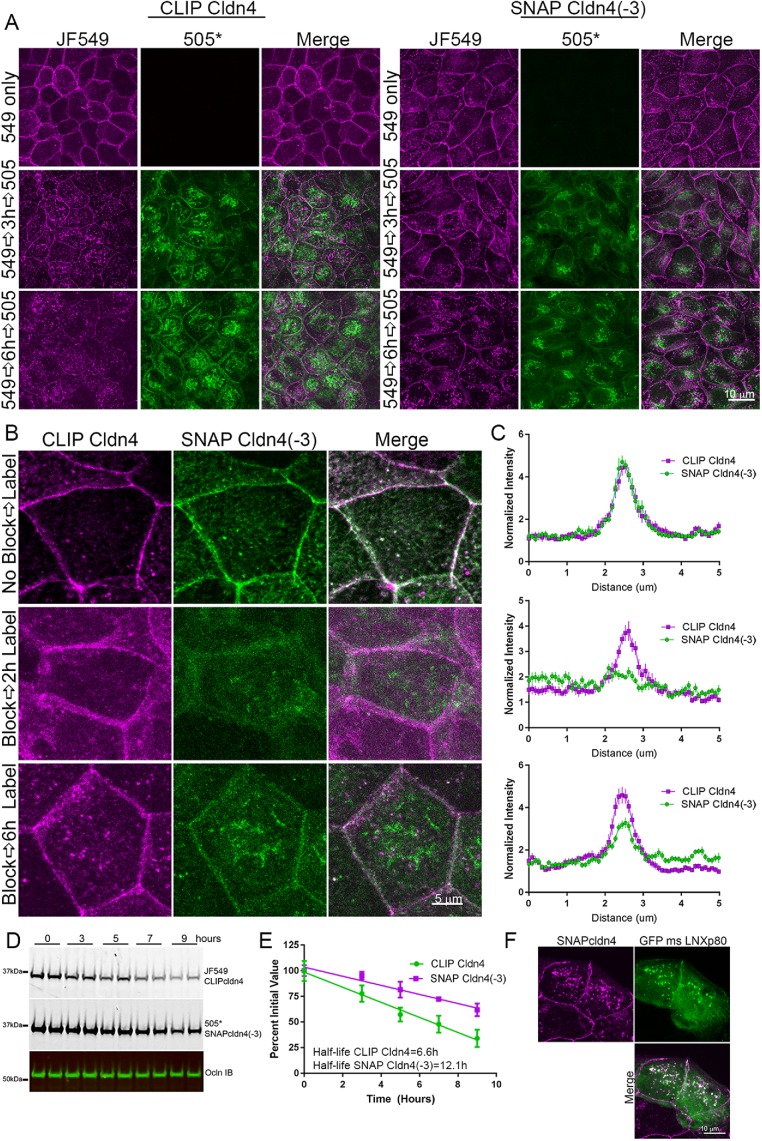
FIGURE 7:
Cldn4 C-terminal amino acids are required for efficient biosynthesis and/or trafficking to the plasma membrane. (A) MDCK II cells stably coexpressing CLIP cldn4 and SNAP cldn4(-3) were labeled first only for CLIP cldn4 localization with either JF549 CLIP-ligand, followed by CLIP-cell block and CLIP-cell 505* (left panels) and second, only for SNAP cldn4(-3) using JF549 SNAP-ligand, SNAP-cell block, and SNAP-cell 505* and fixed at 0, 3, and 6 h (right panels). Fluorescence analysis shows clear lateral membrane staining of new 505*-labeled cldn at 3 h only for full-length cldn4. (B) When SNAP- and CLIP-tagged cldns in coexpressing cells are labeled in the same cell with JF549 CLIP cell ligand and SNAP-cell 505* before and after blocking, old cldns localize identically, independent of the presence of the three C-terminal amino acids, but new CLIP cell cldn appears at cell contacts before SNAP cell cldn4(-3). This finding is quantified by line scanning in C. (D) Comparison of decay in fluorescent JF549-labeled CLIP cldn4 and SNAP-cell 505* SNAP cldn4(-3) in the same cells reveals that SNAP cldn4(-3) is more stable than full-length cldn4; these results are quantified in E. (F) Coexpression of GFP-LNXp80 and SNAP cldn4 reveals colocalization in intracellular vesicles.