Figure 2. Active Site of Human SQOR.
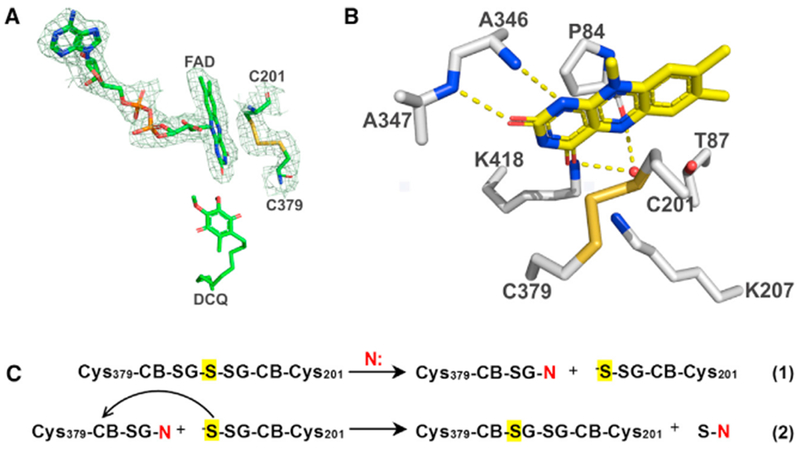
The redox-active site in SQOR (A and B) and the proposed mechanism for conversion of thiocystine-containing enzyme to a catalytically active cystine form (C). The 2Fo – Fc electron density omit map around FAD and the active-site cysteines in (A) is drawn at 1.0 σ. Hydrogen bonds in (B) are indicated by dashed yellow lines. Thiocystine-containing enzyme is converted to a catalytically active cystine form by reaction with a sulfane sulfur acceptor (N: = sulfite or glutathione) present in assay mixtures (C). The proposed activation mechanism results in the retention of the central sulfane sulfur in thiocystine (highlighted in yellow) in the active cystine form of SQOR. See also Figures S1–S3, S6, and S7.
