Figure 6. Substrate Access to the H2S-Oxidizing Active Site.
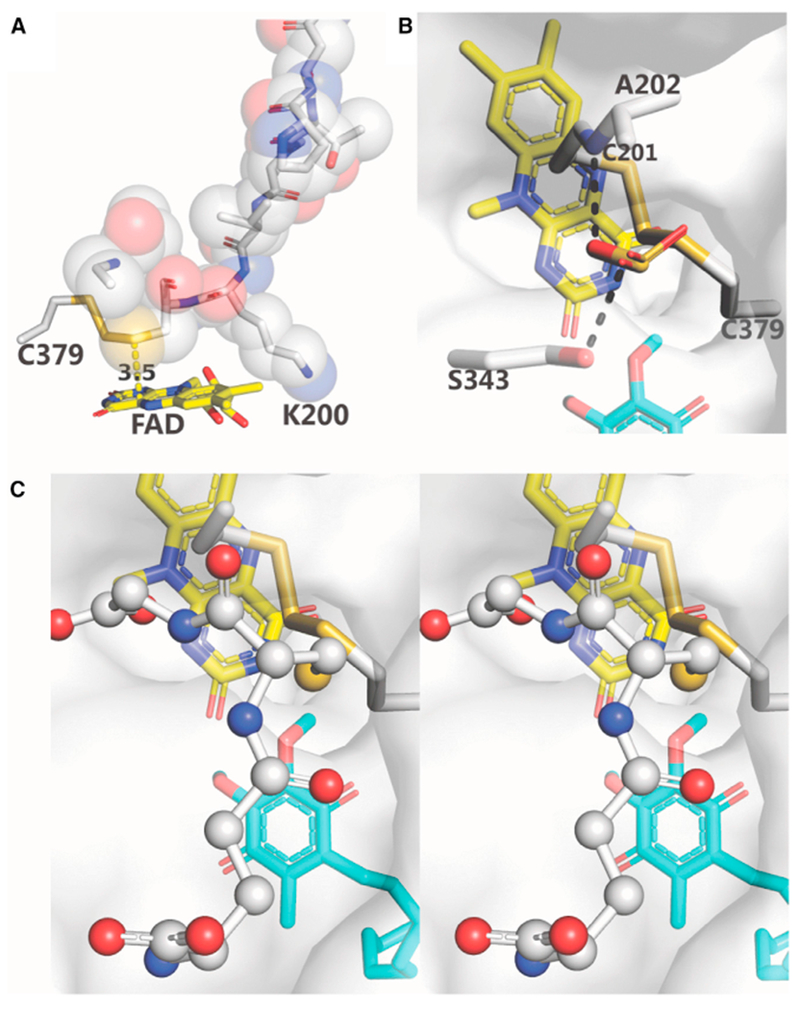
(A) The loop, P195-G203, is shown in sticks and spheres; Cys379 and FAD are shown in sticks. The distance between Cys201:SG and FAD:C4a (3.5 Å) is indicated. Lys344 (not shown) is behind V199-K200.
(B and C) Mono and wall-eye stereoviews of the docking of sulfite (B) and glutathione (C), respectively, to a proposed binding site. The semitransparent protein surface is colored white. Hydrogen bonds are indicated by dashed black lines. Sulfite, FAD, DCQ, and amino acid residues are shown as sticks. Glutathione is shown as ball and sticks.
See also Figures S1 and S8.
