Abstract
Resurgence is the increase in performance of an extinguished instrumental (operant) response that accompanies the extinction of a response that has been reinforced to replace it. Resurgence may involve processes that are relevant for understanding relapse in applied and clinical settings. While resurgence is known to be a robust phenomenon in human operant extinction, the processes that control it remain unclear. Here we asked whether human resurgence is controlled by processes that are similar to those that have been identified in animals by asking whether two methods that reduce resurgence in animals also reduce it in humans. Participants first learned to make an operant response (R1) for a tangible food reinforcer (O1). In a second phase (Phase 2), R1 was extinguished while a second response (R2) was introduced and reinforced with a virtual monetary reward (USD $0.10 coins; O2). In a test phase, extinction was then introduced for R2 and resurgence of R1 was assessed. In Experiment 1, resurgence that occurred after the treatment just described was attenuated if there had been periodic exposure to R2 extinction during the treatment phase (Phase 2). In Experiment 2, resurgence was prevented when O2, but not O1, was presented noncontingently during the test. The results are among the first to suggest a mechanism underlying resurgence in humans, namely, renewal caused by contextual change. They also provide initial evidence to suggest that resurgence may be the result of common processes in animals and humans.
Keywords: Resurgence, Extinction, Context, Human operant conditioning
Resurgence refers to the increase of an extinguished operant response observed when extinction is introduced for a response that has been reinforced to replace it (Lattal & St. Peter Pipkin, 2009). In a typical laboratory study, a hungry rat is first reinforced for emitting an operant response (e.g., lever pressing; R1). In a second phase, emitting R1 is no longer reinforced (extinction) but the rat can emit a different response (R2) to be reinforced. In a final (test) phase, extinction is introduced for R2 and the rat emits R1 again. Resurgence was first studied in animals (e.g., Leitenberg, Rawson, & Bath, 1970), and has since been documented across several responses and species (e.g., da Silva, Cançado, & Lattal, 2014; Leiving & Lattal, 2003; Mulick, Leitenberg, & Rawson, 1976; Nevin et al., 2016). Several authors have suggested that studying resurgence in the laboratory can uncover processes that are relevant for understanding relapse after clinical intervention in humans (e.g., Bouton, 2014; Kestner & Peterson, 2017). For example, differential reinforcement of alternative behavior (DRA) is an intervention for problematic or potentially-harmful behaviors emitted by individuals with developmental disabilities (Petscher, Rey, & Bailey, 2009; Wacker et al., 1990). In DRA, a problem behavior is extinguished while a new response is reinforced to replace it. Since this is the treatment studied in experiments on resurgence, resurgence it is directly relevant to lapse and relapse after DRA treatments (Bouton, 2014; Liddon, Kelley, & Podlesnik, 2017; Nevin & Wacker, 2013).
Resurgence belongs to a collection of phenomena (renewal, reinstatement, spontaneous recovery, rapid reacquisition) that illustrate that extinction does not change behavior permanently. Extinction’s impermanence may be due to the fact that, in operant extinction, the behavior decreases because making the response without the reinforcer allows the organism to learn to inhibit it (e.g., Bouton, Trask, & Carranza-Jasso, 2016). It is clear that whatever new learning is involved in extinction, it is especially context specific (see Bouton, 2017, 2019; Trask, Thrailkill, & Bouton, 2017, for recent reviews). Relapse can thus occur because context-specific extinction learning fails to generalize from the extinction context to new contexts (Bouton, 2002; Bouton, Winterbauer, & Todd, 2012). An example is the “renewal effect” (Bouton & Bolles, 1979; Bouton, 2019). In this effect, an operant response that is reinforced in the presence of a distinct context (olfactory, visual, and tactile cues; Context A) is then extinguished in the presence of a second set of distinct cues (Context B). The response increases (renews) when it is tested back in Context A under continued extinction. Importantly, renewal is also observed if the extinguished response is tested in a third context (Context C; ABC renewal), or in a second context if extinction took place in the same context as training (Context A; AAB renewal; e.g., Bouton, Todd, Vurbic, & Winterbauer, 2011; Todd, 2013). The ABC and AAB forms of renewal suggest that removal from the extinction context is sufficient for behavior to renew. This implies that renewal is at least partly the result of the context-specific property of extinction learning.
Renewal provides an underlying framework for understanding resurgence. Specifically, resurgence can be viewed as an example of the ABC renewal effect (Winterbauer & Bouton, 2010; Trask, Schepers, & Bouton, 2015). Note that background (olfactory, visual and tactile) contextual stimuli are similar across phases of the typical resurgence procedure. Yet, extinction of R1 occurs in the context of reinforcers for R2 during Phase 2, and extinction of R2 in the test changes the context again (Bouton & Trask, 2016; Trask, Keim & Bouton, 2018). This view accepts the idea that reinforcers can function as discriminative stimuli, and is consistent with other research suggesting that many types of stimuli, including reinforcers, can function as a “context” for extinction learning (Bouton, 2019). In resurgence, R1 extinction learning is specific to the context of reinforcers for R2, and this learning does not generalize to the new context of R2 extinction in the test (Trask et al., 2015; 2018). Resurgence is therefore arguably a result of the same process responsible for renewal, reinstatement, spontaneous recovery, and rapid reacquisition (e.g., Bouton, 2014, 2017, 2019).
The contextual account of resurgence makes unique predictions about variables that can reduce its strength. Put generally, it suggests that increasing the similarity of Phase 2 to the conditions of testing will reduce the resurgence effect. Consistent with this idea, a number of studies have found that extinction of R2 during treatment (Phase 2) reduces resurgence of R1 in the test, perhaps by making treatment and test phases more similar. For example, gradually decreasing (or thinning) the rate of reinforcement for making R2 allows more and more nonreinforced R1 responses to occur in the absence of R2 reinforcers. Schepers and Bouton (2015; see also Sweeney & Shahan, 2013) found that thinning the reinforcement rate for R2 reduced resurgence. Interestingly, increasing the reinforcement rate for R2 over Phase 2 after an initially low rate (“reverse thinning”) also reduced resurgence (see also Bouton & Schepers, 2014). Therefore, the reduction in resurgence was not due to thinning making the transition from Phase 2 to the test less discriminable. Instead, exposure to R2 extinction allowed rats to learn extinction of R1 in a context that was similar to the test. To further test this hypothesis, Schepers and Bouton (2015) also compared resurgence in rats that received either the typical Phase 2 treatment or a modified Phase 2 in which each session in which R2 was reinforced alternated with a session in which R2 was extinguished. Experience with alternating R2 extinction sessions attenuated resurgence in comparison to a control group that had R2 reinforced at the same overall average rate in every Phase 2 session (see also Trask et al., 2018).
Additional support for the context account of resurgence comes from other experiments with rats. For example, Bouton and Trask (2016) examined whether the discriminative properties of the reinforcer could be used to attenuate the effect. They found that noncontingent presentations of the R2 reinforcer (O2) during the test, but not the R1 reinforcer (O1), resulted in a near-complete reduction in resurgence. According to the context view, delivering O2 in the test increased the similarity of the test to Phase 2, thus allowing R1 extinction to generalize to the test. More recently, Trask et al. (2018) replicated and extended the approach to show that only the Phase-2 reinforcer (O2), but not an equally familiar reinforcer (O3) that had not been associated with either R1 conditioning or extinction, eliminated resurgence of R1. Overall, the results from animal experiments thus strongly suggest that generalization from Phase 2 to the test is an important target for interventions that aim to reduce resurgence.
Most of what is known about the factors that underlie resurgence and their theoretical implications is based on research in animals (see Trask et al., 2015 for a review). At present, it is unclear whether the variables and mechanisms that control resurgence in animals also apply to human behavior. Resurgence has been demonstrated with simple operant responses in humans (Alessandri, Lattal, & Cançado, 2015; Bolívar, Cox, Barlow, & Dallery, 2017; Marsteller & St. Peter, 2012; Smith, Smith, Shahan, Madden, & Twohig, 2017; Sweeney & Shahan, 2016). And in applied research settings, it has also been demonstrated with problem behavior (Hoffman, & Falcomata, 2014; Lieving, Hagopian, Long, & O’Connor, 2004; Volkert, Lerman, Call, & Trosclair-Lasserre, 2009; Wacker, Harding, Morgan, Berg, Scheiltz, Lee, & Padilla, 2013), care-giving responses (Bruzek, Thompson, & Peters, 2009), and rule-following behavior (Dixon & Hayes, 1998). Although such a range of examples suggests that resurgence is a robust phenomenon in humans, most studies with humans have sought to demonstrate resurgence, rather than explore its underlying causes and controlling variables. In a relatively rare example of a controlling variable that has been identified, high reinforcement rates in Phase 2 support more resurgence in humans than lower rates (Smith et al., 2017). However, such a finding is consistent with several theoretical accounts (Shahan & Craig, 2017; Shahan & Sweeney, 2011; Trask et al., 2015). The present experiments developed a new procedure for studying resurgence in humans and began to isolate the theoretical mechanisms that control it. Specifically, we asked whether methods that increase generalization between Phase 2 treatment and testing could decrease it, as it does in animals. Experiment 1 examined alternating exposure to R2 extinction in Phase 2 as a means to attenuate resurgence of R1 (cf. Schepers & Bouton, 2015). Experiment 2 then compared resurgence of R1 when O1 versus O2 was presented noncontingently during the test (cf. Bouton & Trask, 2016). The results again support a contextual account of resurgence, and further connect the processes that determine resurgence in humans with those that control it in animals.
Experiment 1
As noted above, exposure to R2 extinction during Phase 2 can increase the ability of R1 inhibition to generalize to the test and therefore weaken resurgence (Schepers & Bouton, 2015; Trask et al., 2018). Experiment 1 therefore examined the effect of exposure to R2 extinction in Phase 2 on resurgence with human participants. The design of Experiment 1 is presented in Table 1. Participants first learned to emit an instrumental response that was motivated by food. The method was inspired in part by recent studies with real food outcomes that studied Pavlovian-instrumental interactions (e.g., Lovibond & Colagiuri, 2013; Morris, Quail, Griffiths, Green, & Balleine, 2015; Watson, Wiers, Hommel, & de Wit, 2014). Presses on a keyboard button (R1) sometimes resulted in the presentation of a snack-food image on the computer screen (O1). The images corresponded to actual reinforcers that were later consumed when they were presented by an experimenter. After acquiring R1, every participant then received a treatment phase (Phase 2) and a test. In Phase 2, R1 was placed on extinction for a total of nine 1-min blocks, and a new response (R2) that was incentivized by monetary reinforcement was introduced to replace it. We chose to use a qualitatively different outcome for R2 in order to create a parallel to applied situations that arrange monetary rewards contingent on replacement behaviors (e.g., Bickel, Moody, & Higgins, 2016). For Group Constant, a virtual monetary reinforcer (images of a $0.10 USD coin; O2) was contingent on pressing a second button (R2) in every block. For the other group (Group Alternate), the monetary O2 was also contingent on R2, but only during alternating (odd-numbered) blocks. During the even-numbered blocks, both R1 and R2 underwent extinction. In a final test, extinction was then introduced for R2 as well as R1 in both groups. We expected resurgence of R1 in Group Constant during this test. However, if exposure to R2 extinction during the treatment phase enhances the ability of R1 inhibition to generalize to the test, then resurgence of R1 should be weaker following exposure to the R2 extinction in Group Alternate.
Table 1.
Experimental Designs
| Group | Phase 1 | Phase 2 | Test | |
|---|---|---|---|---|
| Experiment 1 | ||||
| Constant | R1-O1 (VI 12 s) | R1: Ext | R1: Ext | |
| R2: Ext | R2-O2 (VI 6 s) | R2: Ext | ||
| Alternating | R1-O1 (VI 12 s) | R1: Ext | R1: Ext | |
| R2: Ext | R2-O2 (VI 3 s; Odd blocks) Ext (Even blocks) | R2: Ext | ||
| Experiment 2 | ||||
| O1 | R1-O1 (VI 12 s) | R1: Ext | R1: Ext | O1 (VT 12 s) |
| R2: Ext | R2- O2 (VI 12 s) | R2: Ext | ||
| O2 | R1-O1 (VI 12 s) | R1: Ext | R1: Ext | O2 (VT 12 s) |
| R2: Ext | R2-O2 (VI 12 s) | R2: Ext | ||
| Resurgence | R1-O1 (VI 12 s) | R1: Ext | R1: Ext | |
| R2: Ext | R2-O2 (VI 12 s) | R2: Ext |
Note. Reinforcers always consisted of a 1-s presentation of a snack (O1) or $0.10 coin (dime; O2) image. R1 represents the reinforced button during Phase 1. R2 represents the reinforced button during Phase 2. The two buttons were always available. VI = variable interval; VT = variable time; Ext = extinction
Method
Participants
Fifty-seven undergraduate students (44 females) enrolled in introductory psychology courses at the University of Vermont (UVM) participated for course credit. Students signed up anonymously to participate via a recruitment website maintained by the Department of Psychological Science. Students did not have prior experience with research or greater than introductory knowledge of psychology, and were instructed not to eat for 3 hours prior to their appointment. Screening excluded students who reported food allergies. Students ranged in age from 18 to 26. Each participant provided informed consent, and the UVM Institutional Review Board approved all procedures and materials.
Materials and Apparatus
All procedures took place in a room that contained a table with a computer (Dell Optiplex 755), 43-cm (diagonal) monitor, keyboard, and mouse. The keyboard was positioned 19 cm from the edge of the table, and the monitor was positioned 47 cm from the edge, with the bottom 15 cm from the table’s surface. Yellow stickers were affixed to the “M” and “Z” keys on the QWERTY key board. These active keys were 11.5 cm apart (center-to-center). The same yellow stickers were placed on “X”, “F”, “G”, and “N”, but were drawn upon with black marker (inactive keys). A blue sticker was affixed to the “V” button, which was used to advance the task (see below). Button presses were defined as the press and release of key. Button presses were recorded and experimental events were controlled by programs written with Microsoft Visual Studio 2013. In addition to instructions and counts of outcomes earned, the program could display two cartoon images of a vending machine (white on black background). On the screen, the vending machines measured 5.5 cm × 10 cm (w × h). A vending machine could be displayed on either side of the screen positioned 10 cm from the right or left side, 12 cm from the bottom, and 17.5 cm from the top of the screen. An empty bowl for snacks was placed to the right of the computer. Snack food reinforcers (M&M’s, Wavy Lay’s potato chips, or Bare Fuji Red apple chips) were present in small bowls arranged in front of their identifying containers on a second table located on the wall behind the computer table. A fourth bowl contained approximately $2.00 USD in $0.10 coins (dimes). Images of the snack foods in bowls or a dime could be presented on the screen as reinforcers. When displayed, the images were centered on the screen 17.5 cm from either side and 7.2 cm from the top. Snack images measured 5 cm × 4 cm (w × h) and the dime measured 3.8 cm in diameter.
Procedure
Basic demographic information, including age and gender was collected prior to introduction to the experimental room. Participants were directed to the content of the bowls on the second table and encouraged to sample the snacks before taking a seat in front of the computer. Once seated at the computer, participants rated their current level of hunger and the pleasantness of the three snack foods on a 7-point Likert scale. The ensuing experimental task was divided into two sequential stages: In Part 1, the participant could consume snacks presented by the experimenter (see below), and in Part 2 s/he worked on the task without interacting with the experimenter. Participants were asked to rate their level of hunger a second time prior to starting Part 2. If hunger level was rated below “4” at either assessment, the participant was excused from the study and received credit for participation. Participants who completed all behavioral and questionnaire measures did so in a single visit to the laboratory that lasted approximately 40 min.
Phase 1 (R1 conditioning).
In an initial training phase, participants could press a button to earn their highest-rated snack with periodic opportunities to consume the snack. The task consisted of 1-min presentations (blocks) of a vending machine image on the computer monitor. Participants were instructed to use only one finger from their dominant hand to press buttons. A brief practice period oriented participants to the vending machine task. An experimenter read: “This is a vending machine. You can steal snacks from it by pressing the yellow buttons. You will know which is the right button because it will make something happen. You are free to press any of the buttons at all times, but please only use one finger at a time to press.” Participants were then allowed to press the buttons and observe the effects with the experimenter present. A press on the reinforced button resulted in a preferred snack image; presses on other buttons had no consequence. Once the participant made a press to R1 and observed the snack image, the experimenter read: “It is up to you to press the buttons and get as many snacks as you want. You are free to press as much or as little as you want, as fast or as slow as you want, and you will get any of the snacks you steal from the vending machine”. The experimenter then initiated the program and left the room. Aside from a description of how the experimenter would deliver the snacks between blocks, no other instruction was given, and the experimenter did not answer questions related to the task when delivering the snacks. For half the participants, the vending machine was presented on the right side of the screen and the yellow-sticker covered “M” button was reinforced. For the other half, the vending machine was on the left side of the screen and the yellow “Z” button was reinforced. Within a block, reinforcers were arranged according to a variable interval (VI) 12-s schedule, with intervals sampled randomly from a list of ten intervals generated with the method of Fleshler and Hoffman (1962). A reinforcer consisted of a 1-s presentation of an image of the snack. The vending machine image disappeared during the snack image presentation. At the end of a block, the vending machine was replaced with text indicating that the participants could now consume the number of snacks earned in the preceding block. At this point, the experimenter re-entered the room to deliver the snacks into the bowl next to the computer. Participants received one snack for every two images earned in the preceding block. This procedure was intended to reduce satiation (Colagiuri & Lovibond, 2015). The experimenter exited the room after delivering the snacks. Instructions on the screen prompted participants to press the blue “V” button in order to advance to the next block. This procedure was repeated for eight 1-min blocks.
After a brief (approximately 5-min) break, the participant was informed of the opportunity to press buttons to earn more snacks from the vending machine. Instructions indicated that participants would get all the snacks they earned, but not until the end of the experiment. The change in procedure was necessary to reduce contact between the participant and the experimenter during the crucial phases of the experiment. Previous studies have established this method as able to maintain operant responding while minimizing the interaction between participant and experimenter (Prévost, Liljeholm, Tyszka, & O’Doherty, 2012; Quail, Morris, & Balleine, 2017; Quail, Laurent, & Balleine, 2017).
The remaining blocks of the session were separated by a 4-s presentation of text indicating the number of reinforcers earned in the preceding block, followed by a 4-s presentation of a fixation cross in the center of the computer screen. The vending machine was then presented in its usual place (i.e., to the right or left of the fixation cross). The first two blocks were the same as during the initial training phase: One vending machine was present on the screen and pressing R1 was reinforced according to VI 12 s.
Phase 2 (Response elimination).
Beginning on the third block after the break, and for the remainder of the session, a second vending machine was presented on the screen. For all participants, this coincided with the introduction of extinction for R1. That is, pressing R1 no longer had any programmed consequence. For the next nine 1-min blocks, participants could now press the other yellow-stickered button (R2), which was beneath the new second vending machine. Presses to R2 could earn a second outcome, a 1-s presentation of the image of a dime in the same location as where the snack image had been presented during Phase 1. Both vending machines disappeared during dime image presentations. For half the participants (Group Constant), R2 was reinforced according to a VI 6-s schedule in every block. For the other half (Group Alternate), R2 earned dimes according to VI 3 s on odd-numbered blocks (1, 3, 5, 7, and 9), and extinction was in effect for R2 on even-numbered blocks (2, 4, 6, and 8). This arrangement was intended to maintain a similar overall amount of reinforcement in each group in Phase 2 (cf. Schepers & Bouton, 2015).
Test.
After Phase 2, participants received two 1-min test blocks with the vending machines on the screen and extinction in effect for both responses. There was no change to the stimuli to indicate the change from Phase 2 to the test other than the removal of the dime reinforcers.
After completing the session, participants answered two questions regarding the response-outcome contingencies experienced during instrumental training (e.g., What was the result when you pressed the left/right button?), and three questions regarding their use of a strategy (e.g., Please describe the overall strategy you used throughout the study). These questions were used as a rough index of contingency awareness. All monetary reinforcers were virtual. Participants received any snacks they earned in the second part of the task before exiting but did not receive dimes.
Data analysis
The computer recorded the number of responses made during each 1-min block. Responding on R1, R2, and the control blackened buttons were of interest and are reported here. Prior to statistical analysis, participants were excluded if they had (1.) less than 60% of their button presses to R1 during the final 2 blocks of Phase 1, or (2.) less than 60% of their button presses to R2 during the final 2 blocks of Phase 2. There were no exclusions based on performance in the test. Analyses of variance (ANOVAs) were used to assess differences based on between group (e.g., Phase 2 treatment) and within subject factors (e.g., block). The rejection criterion was set to .05 for all statistical tests. Effect sizes and their confidence intervals are reported for tests with relevance to the hypothesis. Confidence intervals on effect sizes were calculated according to the method suggested by Steiger (2004). A Bayes factor (BF) was calculated using JASP software (JASP Team, 2018) and reported when support for a null result was relevant to our hypothesis.
Results
Out of the 57 potential participants that signed up for the study, 30 met criteria for inclusion in the analysis (15 in Group Constant [12 female] and 15 in Group Alternate [12 female]). Participants were excluded because they did not turn up for the appointment (2), reported a hunger level of less than 4 (11), or did not meet the behavioral criteria of more than 60% of their button presses to R1 in the final blocks of Phase 1 (7) or more than 60% on R2 in the final blocks of Phase 2 (7). All of the included participants were able to describe the R1-O1 and R2-O2 relationships accurately.
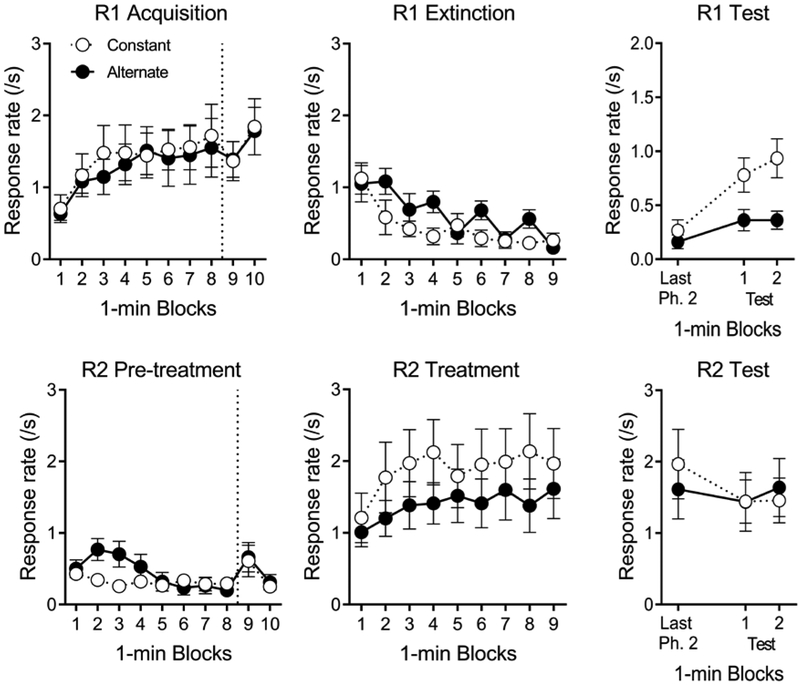
Phase 1.
Participants readily acquired R1 for the consumable O1 in Phase 1 (see top left of Figure 1). A Group (Alternate, Constant) by Block (10) ANOVA found a significant effect of Block, F(9, 252) = 6.50, MSE = 0.44, p < .001. No effect involving Group approached significance, Fs < 1. Though not reinforced, participants did occasionally emit R2 during Phase 1, as suggested by the lower left panel of Figure 1. The same ANOVA applied to R2 found a reliable effect of Block, F(9, 252) = 3.97, MSE = 0.14, p < .001, and a Group by Block interaction, F(9, 252) = 1.97, p = .044; Group Alternate made more R2 responses during the first 4 blocks, but ended the phase making a similar number of presses as Group Constant. There was no reliable effect of Group, F < 1. Group Alternate and Constant obtained a mean of 43.3 (SD = 6.46) and 44.9 (SD = 6.93) presentations of the O1 image in Phase 1. The number of obtained O1 presentations did not differ statistically between the groups, F < 1. Groups Alternate and Constant also made an average of 1.5 (SD = 2.2) and 0.6 (SD = 1.6) responses on the control buttons during Phase 1. Groups did not differ in the number of control-button responses, F(1, 28) = 1.81, MSE = 3.62.
Figure 1.
Results of Experiment 1. Mean response rate (responses per second) on R1 (Top) and R2 (Bottom) across 1-min blocks during Acquisition (Left), Extinction/Treatment (Middle), and the Test (Right). The dashed vertical line separates pretraining blocks that included O1 consumption from the experimental phase. Error bars are the standard error of the mean.
Phase 2.
The center panels of Figure 1 show the results for R1 (top) and R2 (bottom) across blocks of Phase 2. Participants in each group reduced their R1 responding over blocks. Group Alternate showed small increases in R1 responding (resurgences) during the even-numbered blocks, when extinction was in effect for R2. A Group (Alternate, Constant) by Block (9) ANOVA found a significant effect of Block, F(8, 224) = 10.84, MSE = 0.21, p < .001, η2p = .28, 95% CI [.16, .35], and a Group by Block interaction, F(8, 224) = 2.32, p = .021, η2p = .08, 95% CI [.00, .12]. The main effect of Group did not approach significance, F(1, 28) = 1.54, MSE = 1.54. In order to assess whether groups differed in even-numbered blocks versus odd-numbered blocks, a planned Group by Block Type (Odd, Even) by Block (4) ANOVA compared R1 across even- and odd-numbered blocks in the first 8 blocks of Phase 2. The analysis found significant effects of Block, F(3, 84) = 13.59, MSE = 0.33, p < .001, and a Block Type by Block interaction, F(3, 84) = 3.24, MSE = 0.13, p = .026. Importantly, a significant Group by Block Type interaction, F(1, 28) = 13.40, p = .001, η2p = .32, 95% CI [.07, .53], suggested that groups differed in their R1 responding on even-numbered blocks but not odd-numbered blocks. No other effects approached significance, largest F(1, 28) = 1.93, MSE = 1.61. Each group acquired R2 during Phase 2. The same ANOVA applied to R2 found a significant effect of Block, F(8, 224) = 4.09, MSE = 0.35, p < .001. No other effects approached significance, Fs < 1. Groups Alternate and Constant made an average of 0.8 (SD = 1.6) and 0.2 (SD = 0.6) responses on the control buttons, which did not differ, F(1, 28) = 1.85, MSE = 1.46.
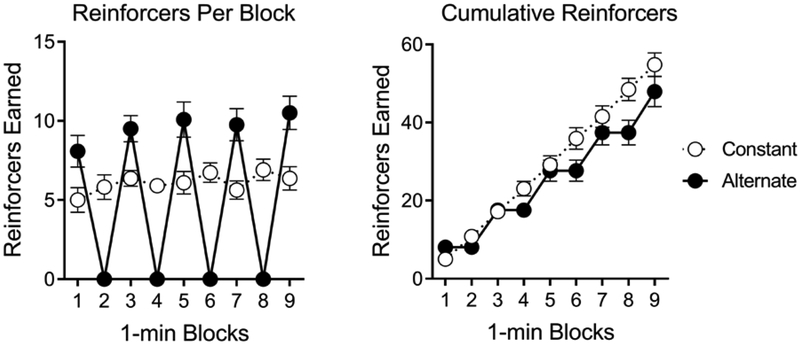
Figure 2 shows the distribution of O2 presentations across blocks of Phase 2 (Left) and the cumulative number of O2 presentations across the entire phase (Right). The number of O2 presentations did not differ between the groups, F(1, 28) = 1.02, MSE = 17.22. A Group by Block ANOVA found a significant effect of Block, F(8, 224) = 45.23, MSE = 4.27, p < .001, and a Group by Block interaction, F(8, 224) = 49.45, p < .001. The same analysis applied to cumulative O2 presentations found a significant effect of Block, F(8, 224) = 440.88, MSE = 15.82, p < .001, and a Group by Block interaction, F(8, 224) = 6.81, p < .001. The main effect of Group was not significant, F(1, 28) = 1.24, MSE = 504.48.
Figure 2.
Reinforcers during Phase 2 in Experiment 1. Mean number of reinforcers obtained in each block of Phase 2 in each group (Left), and mean number of reinforcers accumulated across blocks of Phase 2 in each group (Right). Error bars are the standard error of the mean.
Test.
The right panels of Figure 1 show the last block of Phase 2 followed by the two blocks of the test. Resurgence of R1 (top) is evident in that there was an increase from the last block of Phase 2 to the first block of the test. However, exposure to R2 extinction in Group Alternate reduced resurgence in comparison to Group Constant. A Group by Block (2) ANOVA on the two blocks of testing found a significant effect of Group, F(1, 28) = 7.11, MSE = 0.52, p = .013, η2p = .20, 95% CI [.01, .43]. No effect involving Block reached significance, largest F(1, 28) = 2.09, MSE = 0.04. An additional Group (Alternate, Constant) by Block (Last Phase 2, First Test) ANOVA also assessed the change in responding between the last block of Phase 2 and the first block of testing. There were significant effects of Group, F(1, 28) = 4.35, MSE = 0.23, p = .046, η2p = .13, 95% CI [.00, .36], and Block, F(1, 28) = 14.19, MSE = 0.14, p = .001. The Group by Block interaction did not reach significance, F(1, 28) = 2.72. Planned tests found a significant increase from the last block of Phase 2 to the first block of the Test in Group Constant, F(1, 14) = 9.90, MSE = 0.20, p = .001, η2p = .41, 95% CI [.04, .64], but the increase in Group Alternate failed to reach the conventional criterion of significance, F(1, 14) = 4.33, MSE = 0.07, p = .056. BF = 1.40. Group Alternate remained suppressed relative to Group Constant across the two test blocks.
R2 responding (bottom) was similar across blocks of the test. A Group by Block ANOVA compared R2 responding in the last block of Phase 2 to the first block of the test. The effect of block approached significance, F(1, 28) = 3.99, MSE = 0.46, p = .055, and no other effect was close to significant, Fs < 1. Each group maintain a similar amount of responding across the two test blocks. A Group by Block ANOVA found no reliable effects or interactions, largest F(1, 28) = 1.85, MSE = 0.09. Groups Alternate and Constant made an average of 1.47 (SD = 5.68) and 0.0 (SD = 0.0) responses on the control buttons. Groups did not differ in the number of control-button responses, F(1, 28) = 1.00, MSE = 16.13.
Discussion
Participants acquired R1 for consumable snack reinforcers in Phase 1, and reduced R1 when it no longer produced the reinforcer in Phase 2. They also readily increased R2 when the monetary O2 contingency was introduced in Phase 2. In the test, resurgence occurred in Group Constant, but was significantly weaker in Group Alternate. The results expand the literature on resurgence in humans to include resurgence of responses for food reinforcers. More important, they are the first to test a unique prediction of the contextual theory of resurgence in humans. Consistent with that view and with previous findings in animals (Schepers & Bouton, 2015; Trask et al., 2018), the results suggest that increasing generalization from Phase 2 to the test by including periods of R2 extinction in Phase 2 can attenuate resurgence of human operant responding. This result is not anticipated by a choice-based model of resurgence that predicts no effect of alternating R2 extinction on resurgence of R1 (Shahan & Craig, 2017), or a behavioral momentum-based model that uses the reinforcement rate averaged across Phase 2 to predict similar resurgence in Groups Alternating and Constant (Shahan & Sweeney, 2011). (See the General Discussion for more discussion.)
One difference between the present results and the earlier ones with rats may be worth noting. For Group Alternate, we observed a decreasing and increasing pattern of R1 responding across alternating blocks of R2 reinforcement and nonreinforcement in Phase 2 (see also Schepers & Bouton, 2015; Trask et al., 2018). However, a complementary pattern for R2 responding was not observed, even though it was evident in the earlier rat studies (e.g., Schepers & Bouton, 2015; Trask et al., 2018). The rat-human discrepancy could be due to a number of factors. One especially clear one is that in the rat studies, the subjects received alternating 30-min sessions, whereas here the participants received alternating 1-min blocks. One-min periods of extinction might not be long enough to allow substantial reductions in R2 responding. The greater sensitivity of R1 than R2 to the alternating reinforcement conditions is consistent with animal results suggesting that R2 response rate might have little direct role in the resurgence of R1 (Winterbauer & Bouton, 2010).
Experiment 2
The context hypothesis of resurgence suggests that increasing the similarity of the test context to Phase 2 can also enhance generalization of R1 inhibition to the test and weaken resurgence. With rats, Bouton and Trask (2016) found that noncontingent presentations of the O2 reinforcer from Phase 2 prevented resurgence of R1 in the test. Importantly, this depended on the discriminative property of the reinforcer; noncontingent presentations of the O1 reinforcer from Phase 1 did not suppress a resurgence in R1 responding (see also Trask & Bouton, 2016; Trask et al., 2018). Alternative accounts of resurgence ignore a role for the discriminative properties of the reinforcer and predict that either noncontingent O1 or O2 in the test will reduce resurgence in comparison to an extinction test condition (Shahan & Craig, 2016; Sweeney & Shahan, 2011). Given the theoretical importance of this finding, Experiment 2 examined whether it also occurred in the human operant setting. The design of Experiment 2 is presented in Table 1. Three groups received operant training and extinction in the same manner as in Experiment 1. In the test, two groups (Groups O2 and O1) received noncontingent presentations of the O2 or O1 image, as in Experiment 2. The third group (Group Resurgence) was tested without presentations of either outcome (extinction). The question was whether the discriminative properties of the reinforcer could reduce resurgence by enhancing generalization from Phase 2 to the test. If so, then noncontingent O2 should weaken resurgence in Group O2. In contrast, noncontingent O1 could not enhance generalization from extinction to testing, although it did control for the general effects of reinforcement, broadly defined. If O2 attenuates resurgence, we expected to find reduced responding in the O2 group compared to the resurgence control group. If O1 causes reinstatement, we expected to find enhanced responding in the O1 group compared to the new control. Notice that these outcomes are not mutually exclusive. However, in their analogous rat experiment, Bouton and Trask (2016) found that O2 attenuated resurgence, but that O1 had no facilitating impact on responding that separated it from a resurgence control. The present experiment allowed further opportunity to observe differences in the factors that influence resurgence in animals and humans.
Method
Participants and Apparatus
One hundred and forty-four undergraduate participants (120 females) were recruited using the same recruiting system. Unique subject identifiers prevented the individuals from the previous Experiment 1 from participating in Experiment 2. All participants provided informed consent, and the UVM Institutional Review Board approved all procedures and materials. Materials and apparatus were the same as those described in Experiment 1.
Procedure
After providing consent, participants rated their current level of hunger and pleasantness of the three snacks. As in Experiment 1, both stages of training were then completed sequentially in a single test session.
Phase 1.
The procedure followed that of Experiment 1 except where noted. In the initial training phase, there were 10 one-min blocks in which R1 produced a visual image and actual consumable presentations of the participant’s preferred O1. The participants then earned images of the snack food without actually consuming them for 2 final blocks. At that time, they were informed that they would be able to exchange earned snacks for real ones at the end of the experiment.
Phase 2.
For the remainder of the session, a second vending machine was presented on the screen. Phase 2 consisted of eight 1-min blocks. For all participants, extinction was introduced for R1. All participants also could press the other yellow-stickered button (R2) to earn virtual dimes according to a VI 12-s schedule in every block.
Test.
After Phase 2, participants received two test blocks with the vending machines present and extinction in effect for both responses. For one group of participants (Group O2), noncontingent presentations of the 1-s dime image were presented according to VT 12-s. A second group (Group O1), noncontingent presentations of the 1-s snack reinforcer image from Phase 1 on VT 12-s schedule. A third group (Group Resurgence) was tested in extinction without presentations of either outcome image. Postsession ratings and questionnaire procedures were the same as in Experiment 1.
Results
Out of 144 potential participants that signed up for the study, 28 met criteria for inclusion in the analysis (9 in Group O2 [6 female], 10 in Group O1 [all female], and 9 in Group Resurgence [6 female)]. Participants were excluded because they did not turn up for the appointment (17), reported a hunger level of less than 4 (31), or did not meet the behavioral criteria of more than 60% of their button presses to R1 in the final blocks of Phase 1 (54) or more than 60% on R2 in the final blocks of Phase 2 (14). All of the included participants were able to describe the R1-O1 and R2-O2 relationships accurately.
Phase 1.
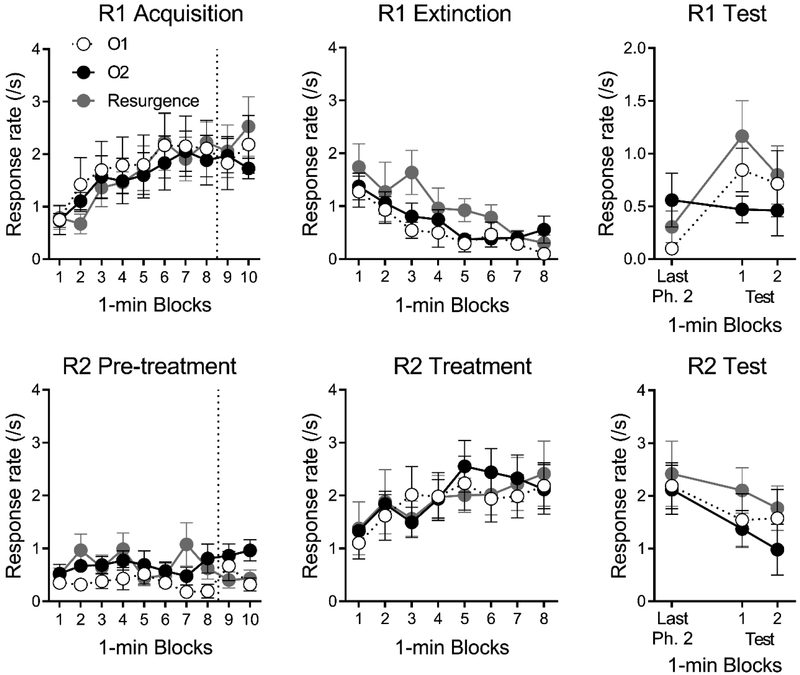
Participants readily acquired R1 for the consumable O1 in Phase 1 (see top left of Figure 3). A Group (O2, O1, Resurgence) by Block (10) ANOVA found a significant effect of Block, F(9, 225) = 10.83, MSE = 0.57, p < .001. No effect involving Group approached significance, Fs < 1. Though not reinforced, participants did occasionally emit R2 during Phase 1, as suggested by the lower left panel of Figure 3. The same ANOVA applied to R2 found no significant effects or interactions, largest F =2.26, MSE = 1.39. Groups O2, O1, and Resurgence obtained a mean of 49.1 (SD = 7.0), 48.0 (SD = 8.8), and 49.2 (SD = 7.3) presentations of the O1 image in Phase 1. The number of obtained O1 presentations did not differ statistically between the groups, F < 1. Groups O2, O1, and Resurgence also made an average of 0.8 (SD = 1.3), 0.8 (SD = 1.6), and 0.3 (SD = 0.7) responses on the control buttons during Phase 1. There was no statistical difference in the number of control-button responses, F< 1.
Figure 3.
Results of Experiment 2. Mean response rates (responses per second) on R1 (Top) and R2 (Bottom) during Acquisition (Left), Extinction (Middle), and Test (Right). The dashed line vertical separates pretraining blocks that included O1 consumption from the experimental phase. Error bars are the standard error of the mean.
Phase 2.
The center panels of Figure 3 show the results for R1 (top) and R2 (bottom) across blocks of Phase 2. Participants in each group reduced their R1 responding over blocks. A Group (O2, O1, Resurgence) by Block (8) ANOVA found a significant effect of Block, F(7, 175) = 9.56, MSE = 0.46, p < .001, and a no other significant effects, largest F = 2.35, MSE = 1.70. Each group acquired R2 during Phase 2. The same ANOVA applied to R2 found a significant effect of Block, F(7, 175) = 4.76, MSE = 0.69, p < .001. No other effects approached significance, Fs < 1. Groups O2, O1, and Resurgence obtained a mean of 34.0 (SD = 6.2), 35.2 (SD = 6.7), and 31.5 (SD = 6.3) presentations of the O2 image in Phase 2. The number of obtained O2 presentations did not differ statistically between the groups, F < 1. Groups O2, O1, and Resurgence made an average of 0.1 (SD = 0.3), 0.1 (SD = 0.3), and 1.1 (SD = 1.9) responses on the control buttons, which did not differ, F(2, 25) = 2.36, MSE = 1.31.
Test.
The right panels of Figure 3 show the last block of Phase 2 followed by the two blocks of the test. Resurgence of R1 (top) in Groups O1 and Resurgence is evident in the increase from the last block of Phase 2 to the first block of the test. However, presentations of O2 in Group O2 reduced resurgence Group O2. A Group (O2, O1, Resurgence) by Block (Last Phase 2, First Test) ANOVA assessed the change in responding between the last block of Phase 2 and the first block of testing. There was a significant effect of Block, F(1, 25) = 11.82, MSE = 0.30, p = .002, η2p = .32, 95% CI [.05, .54], and a Group by Block interaction, F(2, 25) = 4.04, p = .030, η2p = .24, 95% CI [.00, .45]. The Group effect did not reach significance, F < 1. Planned tests found a significant increase from the last block of Phase 2 to the first block of the Test in Groups O1, F(1, 9) = 11.27, MSE = 0.25, p = .008, η2p = .56, 95% CI [.06, .75], and Resurgence, F(1, 8) = 8.02, MSE = 0.42, p = .022, η2p = .50, 95% CI [.01, .73], but there was no increase in Group O2, F < 1, BF = 0.34. There was no evidence to suggest response rates in Groups O1 and Resurgence differed in the first block of the test, F < 1, BF = 0.51.
R2 responding (bottom) was similar across blocks of the test. A Group by Block ANOVA compared R2 responding in the last block of Phase 2 to the first block of the test. There was a significant effect of block, F(1, 27) = 5.60, MSE = 0.809, p = .026, and no other effect was close to significant, Fs < 1. Each group maintain a similar amount of responding across the two test blocks. A Group by Block ANOVA found no reliable effects or interactions, largest F(1, 25) = 1.70, MSE = 0.44. Groups O2 and O1 received a mean of 8.6 (SD = 2.3) and 10.0 (SD = 3.6) presentations of the O2 and O1 image in the test. The number of image presentations did not differ statistically between the groups, F(1, 17) = 1.04, MSE = 9.54. Groups O2, O1, and Resurgence made an average of 0.2 (SD = 0.66), 0.0, (SD = 0.0), and 0.0 (SD = 0.0) responses on the control buttons. Groups did not differ in the number of control-button responses, F(1, 25) = 1.08, MSE = 0.14.
Discussion
Participants again acquired the R1 during Phase 1, and in Phase 2, the extinguished R1 declined while the reinforced R2 increased. Importantly, while Groups O1 and O2 received a similar number of noncontingent reinforcers during testing, the type of reinforcer (O1 vs. O2) determined responding in the test. That is, noncontingent O2 uniquely prevented a resurgence of R1 responding in Group O2. These results extend findings with rats (Bouton & Trask, 2016; Trask et al., 2018) to a laboratory task with human participants. The results continue to support the idea that manipulations that increase the similarity between Phase 2 and testing are effective at reducing the resurgence effect.
It is notable that noncontingent presentations of O1 in Group O1 did not reinstate R1 responding above the level seen in Group Resurgence, which received no reinforcers during the test. The same result was observed in rats using a parallel experimental design (Bouton & Trask, 2016), and were described as possibly due to the fact that the resurgence design includes reinforcer presentations during Phase 2. Presenting the reinforcer during simple extinction can weaken its unique association with conditioning and is known to weaken subsequent reinstatement (e.g., Rescorla & Skucy, 1969; Winterbauer & Bouton, 2011). Bouton and Trask (2016) further suggested that presentations of O1 during the test created a less complete or dramatic change of background context between treatment and testing in comparison to a condition in which reinforcers were absent entirely. Whatever the ultimate explanation, the present results are consistent with the rat observations suggesting that the conditions present in R1 extinction, in this case O2 deliveries, can eliminate R1 resurgence.
General Discussion
The present results suggest that resurgence of operant responding in human participants can be influenced by factors that influence the generalization between treatment and test conditions. In Experiment 1, alternating periods of R2 extinction during Phase 2 had the effect of reducing resurgence that was otherwise observed when R2 had been consistently reinforced at the same overall rate. And in Experiment 2, delivering the O2 reinforcer, but not the O1 reinforcer, noncontingently during the test prevented resurgence. Thus, making treatment conditions more similar to testing conditions (Experiment 1) or testing conditions more similar to treatment conditions (Experiment 2) can both reduce resurgence. Together, the results replicate and extend findings from analogous studies with animals. They thus suggest that similar processes may underlie resurgence in animals and humans.
The present experiments are among the first to test hypotheses regarding the mechanisms that underlie resurgence in humans. Prior studies have tended to focus on whether the effect is merely demonstrable. In contrast, the results of the present experiments are specifically consistent with the context hypothesis of resurgence. As described in the Introduction, the context view conceptualizes resurgence as an instance of the renewal effect (see Trask et al., 2015 for a review). Support for the view has been provided by rat experiments which show that increasing the possible generalization between Phase 2 and the resurgence test can attenuate the resurgence effect (Bouton & Trask, 2016; Schepers & Bouton, 2015; Trask et al., 2018). The present experiments likewise found that manipulations that increased the generalizability of Phase 2 to the test (Experiment 1) or the test to Phase 2 (Experiment 2) can weaken or eliminate resurgence in humans.
The present findings are not anticipated by other accounts of resurgence (Shahan & Craig, 2017; Shahan & Sweeney, 2011). Shahan and Sweeney’s (2011) extension of behavioral momentum theory conceptualizes extinction and DRA as behavioral suppression phenomena (Nevin & Grace, 2000). On this view, resurgence occurs when the suppressive influence of R2 reinforcers is simply removed during the resurgence test. Difficulties with this view have been reviewed elsewhere (e.g., Craig & Shahan, 2016; Trask et al., 2015). Regarding the present experiments, it is not equipped to explain how resurgence is decreased by alternating R2 reinforcement and extinction during treatment compared to a condition that delivered reinforcers at the same, but constant, overall rate (Experiment 1, see also Schepers & Bouton, 2015). It is also not equipped to explain why specific noncontingent presentations of O2 (but not O1) suppressed resurgence (Experiment 2; Bouton & Trask, 2016). Shahan and Craig’s (2017) alternative choice-based account of resurgence has built upon the matching law, which suggests that the relative allocation of behavior to alternative sources of reinforcement will match the relative allocation of reinforcers at those sources (Herrnstein, 1961). On this view, resurgence occurs when the relative value of R1 becomes greater than the relative value of R2 as R2 is extinguished during testing. However, as currently formulated, this theory does not give a role to extinction learning or the discriminative properties of reinforcers suggested by the current experiments, and does not account for the present results nor parallel ones found with rats (Bouton & Trask, 2016; Schepers & Bouton, 2015; Trask et al., 2018; see Trask et al., 2018, for a more detailed analysis of the difficulties for this view). In contrast, the data presented here continue to put resurgence within a theoretical framework that fundamentally emphasizes the context-specificity of extinction learning, a framework that is supported by many years of empirical research (Bouton, 1993, 2017, 2019).
A secondary goal of the present experiments was to arrange a new method to study resurgence in humans in which they earned actual food outcomes and were then encouraged to stop earning them with monetary incentives. In addition to modeling incentivized treatments that are designed to suppress human overeating (e.g., Watson et al., 2014), the use of outcomes with biological relevance arguably brought the method closer to conditions of the animal laboratory. It expanded on a method first developed by Morris et al. (2015), who used a similar vending-machine task and arranged experimenter-delivered food reinforcers that were consumed by the participants (see also Lovibond & Colagiuri, 2013). In the present procedure, however, response-contingent symbols that corresponded to consumable reinforcers were delivered by the experimenter later, between blocks. Thus, the procedure can be viewed as arranging production and exchange contingencies common to token economies (Hackenberg, 2009). To our knowledge, this may be the first application of token-economy methods to study resurgence. It allowed participants to directly experience the food reinforcer, but minimized contact between the participant and experimenter, which allowed participants to experience the instrumental contingencies under conditions similar to those typically arranged for non-human animals in laboratory settings. It is also notable that the present method used a (virtual) monetary outcome as the reinforcer for R2, which may model the monetary incentives often used in applied treatments that incentivize replacement behaviors (e.g., Davis, Kurti, Skelly, Redner, White, & Higgins, 2016).
Recent human laboratory studies of relapse processes (e.g., resurgence, reinstatement) have stressed the importance of distinguishing the return of an extinguished (or “target”) response from a more general increase in behavior induced by changing the reinforcement contingencies (Liggett et al., 2018; Sweeney & Shahan, 2016). For example, in some cases resurgence could result from a general emotional response to the experience of R2 extinction (e.g., frustration) instead of the loss of inhibitory control over R1. This alternative explanation for resurgence has been addressed in animal experiments (Winterbauer & Bouton, 2010). In the present experiments, we found a specific increase in the target response (R1) during resurgence testing; responding did not increase to any of four control buttons. The availability of multiple control buttons, instead of a single one (cf. Cox, Bolivar, & Barlow, 2019; Sweeney & Shahan, 2016), may have reduced any tendency for participants to infer that the third response alternative was the “next” one to be reinforced during the third (test) phase.
A better understanding of processes that underlie resurgence, and relapse in general, may be of interest to clinicians. Resurgence is a form of relapse that can be particularly informative for understanding relapse after interventions that incentivize replacement behaviors, such as functional communication training and DRA-based treatments (St. Peter, 2015; Volkert et al., 2009) and contingency management interventions for individuals with substance use disorders (Bouton, 2014). Accordingly, the present results suggest that clinical methods that aim to increase generalization of treatment to different contexts may reduce the likelihood and perhaps severity of relapse (see also Gámez & Bernal-Gamboa, 2018). Future research is needed to extend this approach outside of laboratory settings. Nonetheless, the present results link the processes that underlie resurgence of simple behaviors from animals to humans. The methods studied here, as well as other methods that reduce resurgence in animals (e.g., reverse thinning, abstinence contingencies; Bouton & Schepers, 2014; Schepers & Bouton, 2015) await application to behavioral interventions in clinical settings.
In summary, resurgence of extinguished operant responding was examined with human participants. The method was unique in that it employed tangible food reinforcers. Three experiments studied whether experimental methods that are known to reduce resurgence in animals could also reduce resurgence in humans under parallel conditions. Exposure to R2 extinction during Phase 2 attenuated resurgence later. And noncontingent O2 reinforcers, but not O1 reinforcers, during testing prevented resurgence. Each result replicates and extends findings with animals to humans, and thus begins to link the processes involved in animal and human resurgence, as well as providing additional support for the contextual account of resurgence.
Acknowledgments
Preparation of this manuscript was supported by NIH Grants R01 DA033123 to MEB and K01 DA044456 to EAT.
Footnotes
Portions of the data presented here were collected in partial fulfillment of the requirements of an undergraduate honors thesis at the University of Vermont by Wesley P. Ameden.
References
- Alessandri J, Lattal KA, & Cançado CR (2015). The recurrence of negatively reinforced responding of humans. Journal of the Experimental Analysis of Behavior, 104, 211–222. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Moody L, & Higgins ST (2016). Some current dimensions of the behavioral economics of health-related behavior change. Preventive Medicine, 92, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolívar HA, Cox DJ, Barlow MA, & Dallery J (2017). Evaluating resurgence procedures in a human operant laboratory. Behavioural Processes, 140, 150–160. [DOI] [PubMed] [Google Scholar]
- Bouton ME (1993). Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin, 114, 80–99. [DOI] [PubMed] [Google Scholar]
- Bouton ME (2002). Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological Psychiatry, 52, 976–986. [DOI] [PubMed] [Google Scholar]
- Bouton ME (2014). Why behavior change is difficult to sustain. Preventive Medicine, 68, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME (2017). Extinction: Behavioral mechanisms and their implications In: Menzel R (ed.), Learning Theory and Behavior, Vol. 1 of Learning and Memory: A Comprehensive Reference, 2nd edition, Byrne JH (ed.). pp. 61–83. Oxford: Academic Press. [Google Scholar]
- Bouton ME (2019). Extinction of instrumental (operant) learning: Interference, varieties of context, and mechanisms of contextual control. Psychopharmacology, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, & Bolles RC (1979). Contextual control of the extinction of conditioned fear. Learning and Motivation, 10, 445–466. [Google Scholar]
- Bouton ME, & Schepers ST (2014). Resurgence of instrumental behavior after an abstinence contingency. Learning & Behavior, 42, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Todd TP, Vurbic D, & Winterbauer NE (2011). Renewal after the extinction of free operant behavior. Learning & Behavior, 39, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, & Trask S (2016). Role of the discriminative properties of the reinforcer in resurgence. Learning & Behavior, 44, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Trask S, & Carranza-Jasso R (2016). Learning to inhibit the response during instrumental (operant) extinction. Journal of Experimental Psychology: Animal Learning and Cognition, 42, 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Winterbauer NE, & Todd TP (2012). Relapse processes after the extinction of instrumental learning: renewal, resurgence, and reacquisition. Behavioural Processes, 90, 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzek JL, Thompson RH, & Peters LC (2009). Resurgence of infant caregiving responses. Journal of the Experimental Analysis of Behavior, 92, 327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colagiuri B, & Lovibond PF (2015). How food cues can enhance and inhibit motivation to obtain and consume food. Appetite, 84, 79–87. [DOI] [PubMed] [Google Scholar]
- Cox DJ, Bolívar HA, & Barlow MA (2019). Multiple control responses and resurgence of human behavior. Behavioural Processes. In press. [DOI] [PubMed] [Google Scholar]
- Craig AR, & Shahan TA (2016). Behavioral momentum theory fails to account for the effects of reinforcement rate on resurgence. Journal of the Experimental Analysis of Behavior, 105, 375–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva SP, Cançado CR, & Lattal KA (2014). Resurgence in Siamese fighting fish, Betta splendens. Behavioural Processes, 103, 315–319. [DOI] [PubMed] [Google Scholar]
- Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, & Higgins ST (2016). A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Preventive Medicine, 92, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MR, & Hayes LJ (1998). Effects of differing instructional histories on the resurgence of rule-following. The Psychological Record, 48, 275–292. [Google Scholar]
- Fleshler M, & Hoffman HS (1962). A progression for generating variable-interval schedules. Journal of the Experimental Analysis of Behavior, 5, 529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gámez AM, & Bernal-Gamboa R (2018). Reinstatement of instrumental actions in humans: possible mechanisms and their implications to prevent it. Acta Psychologica, 183, 29–36. [DOI] [PubMed] [Google Scholar]
- Hackenberg TD (2009). Token reinforcement: A review and analysis. Journal of the Experimental Analysis of Behavior, 91, 257–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ (1961). Relative and absolute strength of response as a function of frequency of reinforcement. Journal of the Experimental Analysis of Behavior, 4, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, & Falcomata TS (2014). An evaluation of resurgence of appropriate communication in individuals with autism who exhibit severe problem behavior. Journal of Applied Behavior Analysis, 47, 651–656. [DOI] [PubMed] [Google Scholar]
- JASP Team (2018). JASP (Version 0.9) [Computer software].
- Kestner KM, & Peterson SM (2017). A review of resurgence literature with human participants. Behavior Analysis: Research and Practice, 17, 1–17. [Google Scholar]
- Lattal KA, & St. Peter Pipkin C (2009). Resurgence of previously reinforced responding: Research and application. The Behavior Analyst Today, 10, 254–266. [Google Scholar]
- Leitenberg H, Rawson RA, & Bath K (1970). Reinforcement of competing behavior during extinction. Science, 169, 301–303. [DOI] [PubMed] [Google Scholar]
- Liddon CJ, Kelley ME, & Podlesnik CA (2017). An animal model of differential reinforcement of alternative behavior. Learning and Motivation, 58, 48–58. [Google Scholar]
- Lieving GA, Hagopian LP, Long ES, & O’Connor J (2004). Response-class hierarchies and resurgence of severe problem behavior. The Psychological Record, 54, 621–634. [Google Scholar]
- Lieving GA, & Lattal KA (2003). Recency, repeatability, and reinforcer retrenchment: An experimental analysis of resurgence. Journal of the Experimental Analysis of Behavior, 80, 217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett AP, Nastri R, & Podlesnik CA (2018). Assessing the combined effects of resurgence and reinstatement in children diagnosed with autism spectrum disorder. Journal of the Experimental Analysis of Behavior, 109, 408–421. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, & Colagiuri B (2013). Facilitation of voluntary goal-directed action by reward cues. Psychological Science, 24, 2030–2037. [DOI] [PubMed] [Google Scholar]
- Marsteller TM, & St. Peter CC (2012). Resurgence during treatment challenges. Mexican Journal of Behavior Analysis, 38, 7–23. [Google Scholar]
- Morris RW, Quail S, Griffiths KR, Green MJ, & Balleine BW (2015). Corticostriatal control of goal-directed action is impaired in schizophrenia. Biological Psychiatry, 77, 187–195. [DOI] [PubMed] [Google Scholar]
- Mulick JA, Leitenberg H, & Rawson RA (1976). Alternative response training, differential reinforcement of other behavior, and extinction in squirrel monkeys (Saimiri sciureus). Journal of the Experimental Analysis of Behavior, 25, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA, & Grace RC (2000). Behavioral momentum and the law of effect. Behavioral and Brain Sciences, 23, 73–90. [DOI] [PubMed] [Google Scholar]
- Nevin JA, Mace FC, DeLeon IG, Shahan TA, Shamlian KD, Lit K, … & Tarver DR (2016). Effects of signaled and unsignaled alternative reinforcement on persistence and relapse in children and pigeons. Journal of the Experimental Analysis of Behavior, 106, 34–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA, & Wacker DP (2013). Response strength and persistence In Madden GJ (Ed.) APA Handbook of Behavior Analysis, Vol. 2 (pp.109–128). American Psychological Association; Washington, D.C. [Google Scholar]
- Petscher ES, Rey C, & Bailey JS (2009). A review of empirical support for differential reinforcement of alternative behavior. Research in Developmental Disabilities, 30, 409–425. [DOI] [PubMed] [Google Scholar]
- Prévost C, Liljeholm M, Tyszka JM, & O’Doherty JP (2012). Neural correlates of specific and general Pavlovian-to-Instrumental Transfer within human amygdalar subregions: A high-resolution fMRI study. Journal of Neuroscience, 32, 8383–8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail SL, Laurent V, & Balleine BW (2017). Inhibitory Pavlovian–instrumental transfer in humans. Journal of Experimental Psychology: Animal Learning and Cognition, 43, 315–324. [DOI] [PubMed] [Google Scholar]
- Quail SL, Morris RW, & Balleine BW (2017). Stress associated changes in Pavlovian-instrumental transfer in humans. The Quarterly Journal of Experimental Psychology, 70, 675–685. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, & Skucy JC (1969). Effect of response-independent reinforcers during extinction. Journal of Comparative and Physiological Psychology, 67, 381–389. [Google Scholar]
- Schepers ST, & Bouton ME (2015). Effects of reinforcer distribution during response elimination on resurgence of an instrumental behavior. Journal of Experimental Psychology: Animal Learning and Cognition, 41, 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahan TA, & Craig AR (2017). Resurgence as choice. Behavioural Processes, 141, 100–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahan TA, & Sweeney MM (2011). A model of resurgence based on behavioral momentum theory. Journal of the Experimental Analysis of Behavior, 95, 91–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BM, Smith GS, Shahan TA, Madden GJ, & Twohig MP (2017). Effects of differential rates of alternative reinforcement on resurgence of human behavior. Journal of the Experimental Analysis of Behavior, 107, 191–202. [DOI] [PubMed] [Google Scholar]
- Steiger JH (2004). Beyond the F test: effect size confidence intervals and tests of close fit in the analysis of variance and contrast analysis. Psychological Methods, 9, 164–182. [DOI] [PubMed] [Google Scholar]
- St. Peter CC (2015). Six reasons why applied behavior analysts should know about resurgence. Mexican Journal of Behavior Analysis, 41, 252–268. [Google Scholar]
- Sweeney MM, & Shahan TA (2013). Effects of high, low, and thinning rates of alternative reinforcement on response elimination and resurgence. Journal of the Experimental Analysis of Behavior, 100, 102–116. [DOI] [PubMed] [Google Scholar]
- Sweeney MM, & Shahan TA (2016). Resurgence of target responding does not exceed increases in inactive responding in a forced-choice alternative reinforcement procedure in humans. Behavioural Processes, 124, 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP (2013). Mechanisms of renewal after the extinction of instrumental behavior. Journal of Experimental Psychology: Animal Behavior Processes, 39, 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, & Bouton ME (2016). Discriminative properties of the reinforcer can be used to attenuate the renewal of extinguished operant behavior. Learning & Behavior, 44, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, Keim C, & Bouton ME (2018). Factors that Encourage Generalization from Extinction to Test Reduce Resurgence of an Extinguished Operant Response. Journal of the Experimental Analysis of Behavior, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask S, Schepers ST, & Bouton ME (2015). Context change explains resurgence after the extinction of operant behavior. Mexican Journal of Behavior Analysis, 41, 187–210. [PMC free article] [PubMed] [Google Scholar]
- Trask S, Thrailkill EA, & Bouton ME (2017). Occasion setting, inhibition, and the contextual control of extinction in Pavlovian and instrumental (operant) learning. Behavioural Processes, 137, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert VM, Lerman DC, Call NA, & Trosclair-Lasserre N (2009). An evaluation of resurgence during treatment with functional communication training. Journal of Applied Behavior Analysis, 42, 145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker DP, Harding JW, Morgan TA, Berg WK, Schieltz KM, Lee JF, & Padilla YC (2013). An evaluation of resurgence during functional communication training. The Psychological Record, 63, 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker DP, Steege MW, Northrup J, Sasso G, Berg W, Reimers T, … & Donn L (1990). A component analysis of functional communication training across three topographies of severe behavior problems. Journal of Applied Behavior Analysis, 23, 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Wiers RW, Hommel B, & De Wit S (2014). Working for food you don’t desire. Cues interfere with goal-directed food-seeking. Appetite, 79, 139–148. [DOI] [PubMed] [Google Scholar]
- Winterbauer NE, & Bouton ME (2010). Mechanisms of resurgence of an extinguished instrumental behavior. Journal of Experimental Psychology: Animal Behavior Processes, 36, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbauer NE, & Bouton ME (2011). Mechanisms of resurgence II: Response-contingent reinforcers can reinstate a second extinguished behavior. Learning and Motivation, 42, 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]