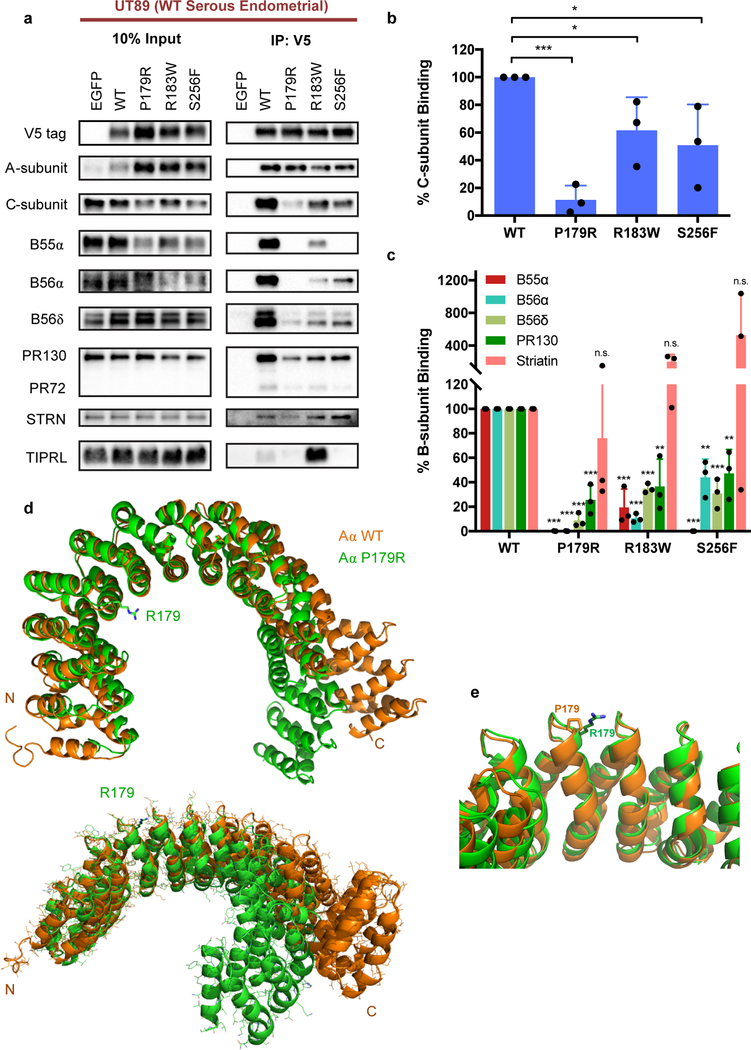
Figure 2. P179R-Aα displays impaired binding to both PP2A B- and C-subunits, which is supported by a global protein conformation change in the resolved crystal structure.
a, Western blots of co-immunoprecipitation (IP) isolates reveals altered interactome with mutant P179R-Aα protein. b–c, Quantification of the percent C- or B-subunit binding relative to WT. Band intensity measurements for subunit protein in the co-IP samples has been normalized to the amount of subunit protein present in the pre-IP input. Data is the average of three independent co-IP experiments (n=3), and represents the mean ± SD. Statistical significance was determined by Student’s t-test (P179R vs. WT). * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, n.s. = not significant. d–e, Overlay of the resolved P179R-Aα crystal structure (green; PDB: 6EF4) with a published WT-Aα crystal structure (orange; PDB: 1B3U) [42]. Crystallographic data collection and refinement details are provided in Supplementary Methods.