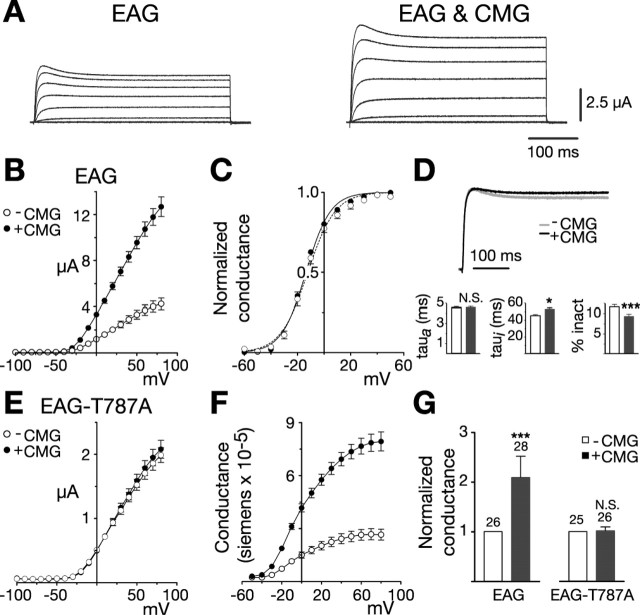
Figure 1.
CMG increases EAG current and conductance. A, Coexpression with CMG increases EAG current amplitudes in Xenopus oocytes. Macroscopic currents recorded in two-electrode voltage clamp from representative oocytes in response to depolarizing voltage steps from -100 to 80 mV in 20 mV increments (holding potential, -80 mV). Linear leakage and capacitative components have been subtracted. Calibration bars apply to both sets of traces. B-G, Dark symbols and bars represent measurements obtained in the presence of CMG. B, Comparison of the average current-voltage relationships obtained for one batch of oocytes (batch refers to oocytes obtained from the same frog) injected with RNA encoding eag (n = 7) or eag together with an excess of cmg (n = 10), as indicated. Currents were elicited by a series of voltage steps from -100 to 80 mV (holding potential, -80 mV). Leak subtraction was performed using a P/4 protocol with pulses of opposite polarity preceding each test pulse (holding potential, -80 mV). C, Normalized conductance-voltage (GV) relationships for wild-type EAG channels, alone (n = 9) or in the presence of CMG (n = 16). For each oocyte, conductance was determined using the relationship G = Itail/(Vtail - EK) and normalized to the maximum conductance. Measurements were made in an extracellular solution containing 25 mm KCl to enhance tail currents. EK was experimentally determined for each oocyte. Boltzmann functions fit to the averaged data had a midpoint and slope of -12.2 mV and 10.3 for EAG and a midpoint and slope of -13.6 mV and 9.2 for EAG in the presence of CMG. D, Top, Scaled representative traces obtained for EAG alone (Ipeak = 3.0 μA) or together with CMG (Ipeak = 4.8 μA). Currents were elicited by a 400 ms pulse to 40 mV from a holding potential of -80 mV. Bottom, Kinetics of EAG currents in the absence and presence of CMG. Three batches of oocytes with a mean fold increase in current amplitude that approximated the mean for all oocytes were selected for kinetic analysis. For each oocyte, activation (left) and inactivation (middle) taus were obtained by fitting two exponentials plus a constant to the first 200 ms of the response to a 400 ms test pulse to 40 mV (holding potential, -80 mV). The percentage of inactivation (inact) was determined by dividing the mean current obtained during the final 10 ms of the pulse by the peak current observed during the pulse. n = 26 and 28 for EAG and EAG with CMG, respectively. The effect of CMG was analyzed using a two-way ANOVA with oocyte batch and CMG as variables (*p < 0.05; ***p < 0.001; N.S., not significant). E, CMG fails to affect EAG-T787A. Experiments were performed as in B. n = 9 and 9 in the absence or presence of CMG, respectively. F, GV relationships for EAG channels expressed alone or together with CMG. Currents shown in B were converted to conductance using the relationship G = Itest/(Vtest - EK). EK was assumed to be -80 mV. G, CMG increases the average whole-cell conductance in oocytes expressing the wild-type, but not mutant, channel. Fold increase in the whole-cell conductance for the oocytes described in D and three batches of oocytes expressing EAG-T787A alone or with CMG. Conductances were determined as described in F in response to a test pulse to 60 mV (holding potential, -80 mV). To normalize for variation in channel expression across different batches of oocytes, the mean conductance in the presence of CMG was normalized to the mean conductance obtained for that batch of oocytes expressing EAG or EAG-T787A alone. The number of oocytes examined for each condition is indicated above each bar. The effect of CMG was statistically analyzed as described in D. Error bars represent SEM.