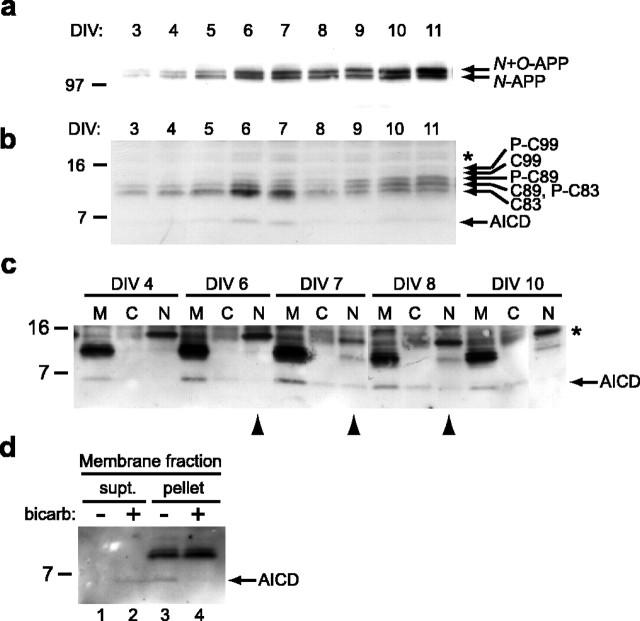
Figure 3.
Temporal regulation of APP products and subcellular localization of AICD in rat primary neurons. a, A similar analysis in rat primary neurons was performed as in Figure 2. Identical sister cultures from the same batch were collected at each time point (DIV) and analyzed for full-length APP. With the same number of cells in the culture of each day, the amount of APP protein increased over time. b, The APP CTFs and AICD fragments were analyzed, demonstrating a highly similar pattern of transient AICD expression as seen in mouse neurons (Fig. 2b). The transient rise in C83 again correlates with the spike in AICD (DIV 6 and 7). c, Baseline AICD is principally associated with membranes, whereas a portion of the induced AICD translocates into the nuclei. Three 10 cm plates at each time point were pooled and subjected to fractionation. After addition of detergent and sonication, samples were immunoprecipitated with C8 and then analyzed by Western blot. M, Membrane; C, cytosol; N, nucleus. The 15 kDa band (*) present in the nuclear fractions is nonspecific, because this band is not confirmed by Western blotting with mouse monoclonal antibody 13G8 (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). d, AICD is loosely associated with membranes. Membrane fractions prepared as in c were washed with buffer or 0.1 m sodium carbonate, pH11. The samples were pelleted, and the resultant supernatant (supt.) and pellet were immunoblotted for the presence of AICD. bicarb, Bicarbonate.