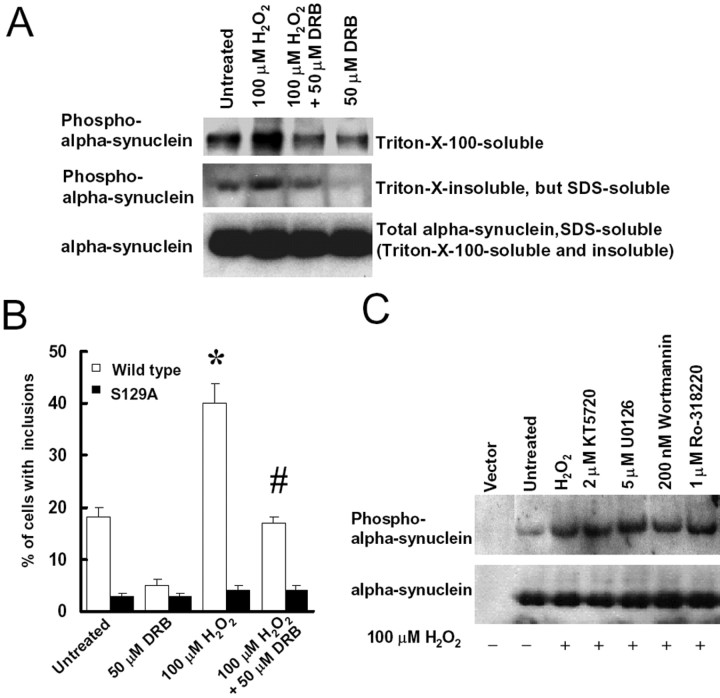
Figure 7.
CK2 inhibitor DRB blocks α-synuclein phosphorylation at S129 and decreases the formation of cytoplasmic inclusions. A, Cells were cotransfected with α-synuclein, myc-synphilin-1, and Flag-parkin for 48 h and then treated with 100 μm H2O2 in either the presence or absence of 50 μm DRB. Twenty-four hours later, Triton X-100-soluble fractions, Triton X-100-insoluble but SDS-soluble fractions, and SDS-soluble fractions (including Triton X-100-soluble and Triton X-100-insoluble fractions) of cell lysates were subjected to anti-α-synuclein and anti-phospho-S129 α-synuclein immunoblotting. The experiment was repeated three times with similar results. B, Cells were cotransfected with myc-synphilin-1, Flag-parkin, and α-synuclein, with or without S129 alterations, for 48 h, followed by 100 μm H2O2 treatment in presence or absence of 50 μm DRB. Cells were collected 24 h later for immunocytochemical assay with anti-phospho-S129 and anti-ubiquitin antibodies. Cells with ubiquitin-positive and phospho-α-synuclein-positive inclusions were counted. Data are shown as the means ± SE for three separate experiments performed in duplicate. *p < 0.05 versus untreated cells transfected with wild-type α-synuclein, myc-synphilin-1, and Flag-parkin. #p < 0.05 versus 100 μm H2O2-treated cells transfected with wild-type α-synuclein, myc-synphilin-1, and Flag-parkin. C, Cells were cotransfected with α-synuclein, myc-synphilin-1, and Flag-parkin for 48 h and treated with 100 μm H2O2 in presence or absence of 2 μm KT5720, 5 μm U0126, 200 nm wortmannin, or 1 μm Ro-318220. Cell lysates were prepared 24 h later and were subjected to anti-α-synuclein and anti-phospho-S129 α-synuclein immunoblotting. The experiment was repeated three times with similar results.