Abstract
Recent studies support the hypothesis that soluble oligomers of amyloid β-peptide (Aβ) rather than mature amyloid fibrils are the earliest effectors of synaptic compromise in Alzheimer's disease. We took advantage of an amyloid precursor protein-overexpressing cell line that secretes SDS-stable Aβ oligomers to search for inhibitors of the pathobiological effects of natural human Aβ oligomers. Here, we identify small molecules that inhibit formation of soluble Aβ oligomers and thus abrogate their block of long-term potentiation (LTP). Furthermore, we show that cell-derived Aβ oligomers can be separated from monomers by size exclusion chromatography under nondenaturing conditions and that the isolated, soluble oligomers, but not monomers, block LTP. The identification of small molecules that inhibit early Aβ oligomer formation and rescue LTP inhibition offers a rational approach for therapeutic intervention in Alzheimer's disease and highlights the utility of our cell-culture paradigm as a useful secondary screen for compounds designed to inhibit early steps in Aβ oligomerization under biologically relevant conditions.
Keywords: Alzheimer's disease, amyloid β-peptide, oligomers, LTP, size exclusion chromatography, fibrillogenesis
Introduction
The onset of Alzheimer's disease (AD) is remarkably insidious, with impairment of short-term memory the earliest detectable symptom. Analysis of cortical biopsies from patients with symptomatic AD indicates that synaptic injury may occur early in the disease process (Davies et al., 1987). Strong evidence suggests that the amyloid β-peptide (Aβ) plays a central role in disease pathogenesis (Hardy and Selkoe, 2002) and that Aβ-induced synaptic dysfunction may underlie early memory loss (Selkoe, 2002).
We showed recently that maintenance of hippocampal long-term potentiation (LTP) is inhibited by culture medium containing naturally secreted human Aβ oligomers (Walsh et al., 2002a; Wang et al., 2004) and that the block of LTP was attributable to soluble Aβ oligomers (Walsh et al., 2002a). These findings are in accord with studies demonstrating that soluble, prefibrillar aggregates of synthetic Aβ also confer neurotoxicity (Roher et al., 1996; Lambert et al., 1998; Hartley et al., 1999) and that behavioral and morphological alterations are found in amyloid precursor protein (APP) transgenic mice before amyloid deposition (Hsia et al., 1999; Mucke et al., 2000). Importantly, studies of human brain demonstrate a strong correlation between the levels of soluble Aβ and severity of dementia (Lue et al., 1999; McLean et al., 1999; Wang et al., 1999). SDS-stable dimers and trimers detected in the buffer-soluble fraction of human cortex (McLean et al., 1999) are strikingly similar to the SDS-stable Aβ oligomers detected in the lysates and conditioned medium (CM) of cultured cells (Podlisny et al., 1995; Morishima-Kawashima and Ihara, 1998). The Aβ species that we detected in CM were confirmed as bona fide Aβ oligomers by both N-terminal radiosequencing and precipitation with C-terminal-specific antibodies (Podlisny et al., 1995; Walsh et al., 2000). Moreover, their pathogenic relevance is supported by the finding that oligomer formation is increased by expression of AD-causing mutations in APP or presenilin (Xia et al., 1997).
Based on these and other findings, inhibition of Aβ aggregation is a rational target for therapeutic intervention. Therefore, we have characterized the effects on natural Aβ oligomerization of four compounds known to inhibit synthetic Aβ fibril formation or neurotoxicity or both. RGKLVFFGR-OH (OR1) and RGKLVFFGR-NH2 (OR2) are novel nonapeptides that potently inhibit in vitro fibrillogenesis of synthetic Aβ. N,N′-bis(3-hydroxyphenyl)pyridazine-3,6-diamine (RS-0406) prevents and reverses fibril formation and ameliorates synthetic Aβ-mediated toxicity and impairment of LTP (Nakagami et al., 2002a). A structurally related compound, 6-ethyl-N,N′-bis(3-hydroxy-phenyl) [1,3,5]triazine-2,4-diamine (RS-0466), does not inhibit Aβ fibril formation but retains the ability to prevent Aβ-mediated toxicity (Nakagami et al., 2002b).
Here, we show that both RS-0406 and RS-0466 inhibit the intracellular formation of cell-derived Aβ oligomers and that CM from cells treated with RS-0406 or RS-0466 contained reduced oligomer levels and no longer blocked LTP. Using nondenaturing size exclusion chromatography, we physically separated Aβ monomer from low-n oligomers and unambiguously identified Aβ oligomers as the species responsible for perturbing synaptic activity. Using this orthogonal approach, we confirmed that both RS-0406 and RS-0466 inhibit natural oligomer formation. We conclude that the fractionation protocol described here will be of use in further investigating the biological activity of natural Aβ oligomers and that compounds such as RS-0406 and RS-0466 may have utility in treating the earliest stages of synaptic dysfunction in AD.
Materials and Methods
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. RS-0406 (Nakagami et al., 2002a) and RS-0466 (Nakagami et al., 2002b) were obtained from Dr. Yasuhiro Nakagami (Sankyo, Tokyo, Japan) and Dr. Russell Hagan (BTG International, London, UK), respectively. Peptides OR1 and OR2 were synthesized using conventional Fmoc [N-(9-fluorenyl) methoxycarboxyl] chemistry and purified on a Vydac (Columbia, MD) C4 column. Peptide purity (>95%) and identity were confirmed by analytical HPLC and electrospray mass spectrometry.
Aβ peptides. Aβ(1-40) and Aβ(1-42) were synthesized and purified essentially as described previously (Walsh et al., 1997). Peptide mass, purity, and quantity were determined by a combination of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, analytical HPLC, and quantitative amino acid analysis.
Thioflavin T-binding assay. Aβ peptides were dissolved in dimethylsulfoxide (DMSO) to yield protein concentrations of ∼2 mm and then diluted with milliQ water to yield a ∼200 μm stock solution. To initiate reactions, 142.5 μl of 100 mm Tris-HCl, pH 7.4, was added to 150 μl of Aβ stock solution with 7.5 μl of either 10 mm test compound in 10% DMSO or 10% DMSO vehicle alone. Samples were vortexed briefly, aliquots were assayed immediately, and the remainder was incubated at 37°C. At intervals of 24 and 48 h, samples were removed, vortexed for 10 s, and then assayed. Electron microscopy (EM) was performed on samples after 48 h of 37°C incubation.
Thioflavin T (ThT) binding was assessed as described previously (Walsh et al., 1999). Briefly, 25 μl of sample was added to a 1 cm2 cuvette containing 425 μl of water and 500 μl of 100 mm glycine-NaOH, pH 8.5. The cuvette was vortexed, and then 50 μl of 100 μm ThT was added and the solution was vortexed again. Fluorescence was measured at 90, 100, 110, and 120 s after ThT addition. Measurements were made using a Hitachi (Tokyo, Japan) F-4500 fluorescence spectrophotometer with excitation and emission at 446 nm (slit width, 5 nm) and 490 nm (slit width, 10 nm), respectively.
APP-expressing cells. Chinese hamster ovary (CHO) cells stably transfected with a cDNA encoding APP751 containing the Val717Phe familial AD mutation (referred to as 7PA2 cells) were cultured in DMEM with 10% fetal bovine serum (Hyclone, Logan, UT), as described previously (Koo and Squazzo, 1994).
Antibodies. Antibodies to APP and its proteolytic derivatives have been described previously (Walsh et al., 2000). Monoclonal antibody 2G3 was raised to Aβ33-40 and specifically recognizes Aβ species ending at residue 40, whereas monoclonal antibody 21F12 was raised to Aβ33-42 and specifically recognizes Aβ species ending at residue 42. Both 2G3 and 21F12 were kindly provided by Drs. P. Seubert and D. Schenk (Elan Pharmaceuticals, San Francisco, CA). Monoclonal antibodies 6E10 and 4G8 (Signet Pathology Systems, Dedham, MA) recognize epitopes within the human Aβ sequence corresponding to residues 6-10 and 17-24, respectively; R1282 is a high-titer polyclonal antiserum raised to synthetic Aβ1-40 (Haass et al., 1992). Polyclonal antiserum C7 was raised to a peptide encompassing residues 676-695 of the APP sequence and was used to immunodeplete cell lysates of full-length APP and its C-terminal fragments (Walsh et al., 2000).
Treatment of cells with test compounds. Nearly confluent (95-100%) 10 cm2 dishes of 7PA2 cells and their corresponding untransfected parental CHO cell line were washed with serum-free medium (4 ml × 1) and incubated with or without compound in serum-free medium for ∼15 h. CM was then removed and cleared of cells by centrifugation at 200 × g for 10 min at 4°C. Protease inhibitors (final concentration: 5 μg/ml leupeptin, 5 μg/ml aprotinin, 2 μg/ml pepstatin, 120 μg/ml Pefabloc) were added to the supernatant, and this CM was used for immunoprecipitation (IP) or concentrated ∼10-fold using Centriprep YM-3 centrifugal filter devices (Millipore, Waltham, MA). Protease inhibitors, many of which are biologically active, were not added to samples to be used for electrophysiology; however, the absence of protease inhibitors did not substantially alter the detection or the size exclusion chromatography (SEC) elution pattern of Aβ monomer and oligomers.
The concentrations of test compounds were determined empirically; in the case of RS-0406 and RS-0466, the concentrations used (102 and 9.3 μm, respectively) were the maximum tolerated without inducing cytotoxicity. For OR-1 and OR-2, the concentration used (18.5 μm) was determined by the solubility of these peptides. In all cases, the ratios of compound to Aβ were far in excess of those used in experiments in which RS-0406, OR-1, and OR-2 were shown to inhibit in vitro fibrillogenesis (see Fig. 1).
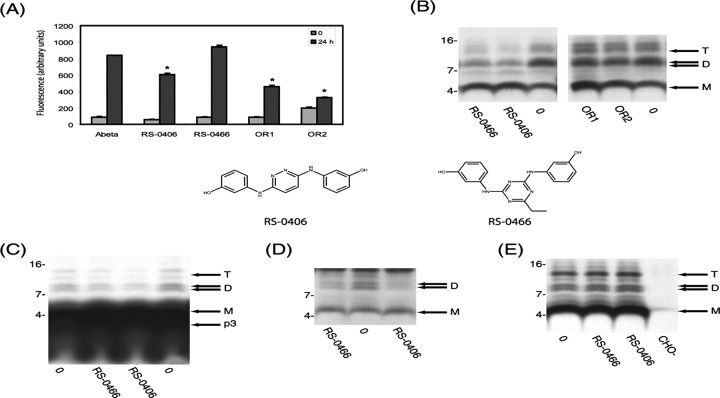
Figure 1.
Hydroxyanaline derivatives RS-0406 and RS-0466 inhibit Aβ oligomerization A, Synthetic Aβ(1-42) was incubated at 100 μm in 50 mm Tris-HCl, pH 7.4, at 37°C with and without 0.25 mm of each test compound, and aliquots were assayed for thioflavin T binding. RS-0406, OR1, and OR2 each significantly decreased in vitro synthetic fibrillogenesis (*p < 0.05). Error bars represent SD. B, 7PA2 cells were grown to near confluence in 10 cm2 dishes and allowed to condition serum-free medium with and without test compounds [(in μm): 102 RS-0406, 9.3 RS-0466, 18.5 OR1, and 18.5 OR2]. After 15 h, the CM was collected, cleared of cells, and analyzed by IP with R1282 and Western blotting with 6E10. Only RS-0406 and RS-0466 decreased natural oligomer levels. M, D, and T designate Aβ monomer, dimer, and trimer. C, 7PA2 cells were grown to near confluence in 10 cm2 dishes as described in B but labeled with [35S]methionine for 15 h with and without RS-0406 or RS-0466. Thereafter, the CM was collected, cleared of cells, immunoprecipitated with R1282, and analyzed by SDS-PAGE and autoradiography. M, D, and T are as in B. Aβ monomer, 5 kDa Aβ, and p3 (the latter being the proteolytic product of the α- and γ-cleavages of APP) do not appear as discrete bands at the exposure time necessary to visualize Aβ oligomers. Again RS-0466 and RS-0406 decreased oligomer levels. D, 7PA2 cells were grown in the presence or absence of RS-0406 or RS-0466, and lysates were prepared and immunoprecipitated with R1282. Both Aβ monomer (M) and dimer (D) bands are readily detected in lysates from untreated cells (0), but the dimer bands are significantly reduced in lysates of cells grown in the presence of RS-0406 or RS-0466. Because 6E10 also detects the more abundant APP C-terminal fragment, C99, the trimer is not discernible. Both compounds decreased the amount of dimers detected intracellularly. E, 7PA2 cells were grown to near confluence in 10 cm2 dishes as described in B and allowed to condition serum-free medium in the absence of any compounds. After harvesting and removing the cells by low-speed centrifugation, the CM was incubated with and without RS-0406 or RS-0466 for 15 h at 37°C. Neither compound altered the pattern of detection of oligomers, thus confirming that these hydroxyanaline compounds do not act by breaking up preexisting oligomers but rather act by inhibiting oligomerization.
Immunoprecipitation/Western blot analysis. A highly sensitive IP/Western blot (wblot) protocol that can readily detect as little as 200 pg of naturally secreted Aβ (Walsh et al., 2000) was used for the detection of natural, secreted Aβ in samples that were not radiolabeled. Samples were immunoprecipitated with the high-affinity antibody R1282 at a dilution of 1:75. After IP, samples were electrophoresed on 10-20% tricine gels and transferred to 0.2 μm nitrocellulose membranes at 400 mA for 2 h. Filters were boiled for 10 min in PBS (Ida et al., 1996) and then blocked overnight at 4°C with 5% fat-free milk in 20 mm Tris-HCl, pH 7.4, containing 150 mm NaCl and 0.05% Tween 20 (TBS-T). After washing the membranes in TBS-T, they were probed with monoclonal 6E10 (1 μg/ml). Bound antibody was visualized with horseradish peroxidase-conjugated donkey anti-mouse Ig (at 1:25,000) (Jackson ImmunoResearch, West Grove, PA) and the ECL+ detection system (Amersham Biosciences, Arlington Heights, IL).
Radiolabeling, immunoprecipitation, and gel fluorography. Nearly confluent (95-100%) 10 cm2 dishes of cells were starved of methionine for 30 min and were then labeled with 750 μCi [35S]methionine for ∼15 h with or without the test compounds used above. Media were harvested and immunoprecipitated (Podlisny et al., 1995). After electrophoresis on 10-20% tricine gels, bands were visualized by gel fluorography.
Size exclusion chromatography. A Superdex 75 10/30 HR column attached to an AKTA FPLC system (Amersham Biosciences, Piscataway, NJ) was used for all experiments and calibrated using unbranched dextran standards of molecular masses: 123,600; 66,700; 43,800; 21,400; 9890; and 4440 (Pharmacosmos, Holbaek, Denmark). Concentrated CM (1 ml) was injected onto the Superdex 75 column and eluted with 50 mm ammonium acetate, pH 8.5, at a flow rate of 1 ml/min. One milliliter fractions were lyophilized, resuspended in 2× SDS-tricine sample buffer (20 μl), boiled for 10 min, and then electrophoresed on 10-20% tricine gels. Proteins were transferred onto 0.2 μm nitrocellulose and used for Western blotting as described above.
Preparation of brain slices. Coronal sections of mouse hippocampus (males and females; 14-35 d of age) were used for all experiments. After asphyxiation with CO2, the brain was rapidly removed and placed in ice-cold oxygenated artificial CSF (ACSF) containing sucrose that contained the following (in mm): 206 sucrose, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 1 CaCl2, 1 MgCl2, 26 NaHCO3, 10 d-glucose, pH 7.4, ∼315 mOsm (Moyer and Brown, 1998). Transverse slices (350 μm thick) were cut and transferred to a holding chamber containing artificial CSF that contained the following (in mm): 124 NaCl, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 2 CaCl2, 26 NaHCO3, 10 d-glucose, pH 7.4, ∼310 mOsm, in which they were allowed to recover for at least 1 h. The slices were then transferred to a recording chamber for submerged slices and continuously superfused at a rate of 3 ml/min (∼22°C). Slices were incubated in the recording chamber for 20 min before stimulation. A closed-loop system containing 15 ml of ACSF was used to minimize the bath volume and hence the volume of CM.
Electrophysiological recordings. Standard electrophysiological techniques were used to record field potentials in the CA1 of hippocampus (Stanton et al., 1987). A unipolar stimulating electrode (World Precision Instruments, Sarasota, FL) was placed in the Schaffer collaterals to deliver baseline stimuli and tetani. A borosilicate glass recording electrode containing ACSF was positioned in the CA1, ∼75-200 μm from the stimulating electrode. The intensity of the stimulus (typically between 10 and 20 μA) was set to obtain 20-30% of the maximal field potential response, and test stimuli were delivered at 0.05 Hz. To induce LTP, four tetani (100 Hz for 1 s) were delivered 5 min apart. Field potential responses were amplified 100× using an Axoprobe 1A. The data were sampled at 10 kHz and filtered at 2 kHz. Traces were analyzed using the LTP Program (www.ltp-program.com). The slope of the field potential was estimated using ∼10-60% of the total response.
For analysis of the effects of 7PA2 and CHO-CM, media were concentrated ∼15-fold using Centriprep YM-3 centrifugal filter devices and frozen at -80°C. On the day of the experiment, concentrated CM samples were diluted 1:15 with ACSF immediately before analysis. For comparison of monomeric and oligomeric Aβ, concentrated 7PA2 or CHO-CM was subjected to SEC as described, and 1 ml fractions were collected. Samples were lyophilized and, on the day of the experiment, reconstituted in 10 ml of ACSF. An aliquot (1.5 ml) of each reconstituted fraction was used for IP/wblot analysis, and the remaining 8.5 ml was used for electrophysiology.
Statistics. The effect of test compounds on Aβ1-42 fibrillogenesis was compared with the no-compound control using an unpaired t test with Welch correction, and the Bonferroni multiple comparison test was used to compare treatment and control EPSP slope at 60 min after highfrequency stimulation (HFS).
Results
A hydroxyanaline derivative and two Aβ-derived peptides decrease synthetic Aβ fibrillogenesis in vitro
We examined four compounds known to alter Aβ fibrillogenesis and/or neurotoxicity. RS-0406 (Fig. 1), a hydroxyanaline compound, has been reported to prevent and reverse synthetic Aβ fibrillogenesis and consequent toxicity, whereas another hydroxyanaline, RS-0466 (Fig. 1), appears not to inhibit synthetic Aβ fibrillogenesis but can prevent its toxicity. OR1 and OR2 are peptides that have sequences similar to the central hydrophobic core of Aβ. OR1 and OR2 are novel compounds that potently inhibit synthetic Aβ fibrillogenesis. We assessed in vitro fibrillogenesis by ThT binding and EM. Preliminary experiments had revealed that under the conditions used, the fibrillogenesis of 100 μm Aβ1-42 reached a plateau by 24 h (data not shown), so the effect of each compound was compared at 0 and 24 h. In agreement with published findings (Nakagami et al., 2002a), RS-0406 caused a significant decrease in the amount of ThT-positive fibrillar material (Fig. 1A) as well as in the number of fibrils detected by EM (data not shown), whereas RS-0466 decreased neither parameter. Indeed, RS-0466 caused a slight increase in ThT binding; this was not attributable to intrinsic fluorescence of RS-0466 or an interaction between RS-0466 and ThT, because the time-zero ThT reading for the no-compound control was indistinguishable from that for the RS-0466-containing sample. In contrast, both OR1 and OR2 caused a substantial decrease in ThT binding (Fig. 1A) and in the amount of fibrils detected by EM (data not shown), thus demonstrating that like RS-0406, both OR1 and OR2 are capable of inhibiting in vitro fibrillogenesis.
The two hydroxyanaline derivatives prevent formation of SDS-stable oligomers of natural Aβ in living cells
We reported previously that the CM of CHO cells stably expressing human APP751 with the V717F FAD mutation (designated 7PA2 cells) contains SDS-stable oligomers of Aβ (Podlisny et al., 1995). The Aβ assemblies we detected in CM have been confirmed as bona fide Aβ oligomers by both N-terminal radiosequencing and precipitation with Aβ40- and Aβ42-specific C-terminal antibodies (Podlisny et al., 1995; Walsh et al., 2000). These oligomers migrate in denaturing gels principally as dimers and trimers, plus a small amount of tetramers (Podlisny et al., 1995; Walsh et al., 2002b). The pathogenic relevance of these natural Aβ oligomers is supported strongly by the finding that oligomer formation is augmented by expressing AD-causing mutations in APP or presenilin that are known to increase Aβ42 production (Xia et al., 1997) and by the demonstration that the oligomer-containing CM can block maintenance of hippocampal LTP both in vivo (Walsh et al., 2002a) and in slices (Wang et al., 2004). Therefore, we tested the effect of compounds known to prevent synthetic Aβ fibrillogenesis in vitro on the formation of these cell-derived Aβ oligomers. Treatment of 7PA2 cells with RS-0406 and RS-0466 decreased detection of oligomers in CM (Fig. 1B), but neither OR1 nor OR2, both of which potently inhibit synthetic Aβ fibrillogenesis (Fig. 1A), altered the quantity of natural Aβ oligomers detected (Fig. 1B). Similarly, a number of other compounds known to potently inhibit in vitro fibrillogenesis had no effect on formation of cell-derived oligomers (data not shown). RS-0406 and RS-0466 decreased the amount of Aβ dimers and trimers detected in the CM, as assessed by our sensitive IP/wblot protocol (Fig. 1B) or by IP/ autofluorography of CM from [35S]methionine-labeled cells (Fig. 1C). By both methods, the level of the monomer band was modestly increased (Fig. 1B).
Because we had shown previously that Aβ oligomerization in these cells appears to be initiated in the subcellular vesicles in which Aβ is generated (Walsh et al., 2002a), we next asked whether RS-0406 and RS-0466 inhibit intracellular oligomerization or rather cause the disassembly of oligomers secreted into the medium. 7PA2 cells were conditioned in the presence or absence of each hydroxyanaline derivative, and whole-cell lysates were prepared and analyzed. Both compounds caused the reduction of intracellular Aβ oligomer levels, whereas Aβ monomer was essentially unchanged, compared with cells treated with vehicle alone (Fig. 1D). In contrast, when RS-0406 and RS-0466 were added to oligomer-containing CM in vitro, the monomer and oligomer patterns were indistinguishable from the CM lacking these compounds (Fig. 1E). Together, these results demonstrate that RS-0406 and RS-0466 can prevent intracellular oligomerization of Aβ but appear not to disassemble the natural oligomers once they are released from the cell.
Conditioned medium from 7PA2 cells treated with RS-0406 and RS-0466 no longer block LTP
In agreement with our recent demonstration that oligomer-containing 7PA2 CM can block LTP in rat hippocampal slices (Wang et al., 2004), we found that 7PA2 CM also caused a dramatic reduction of the maintenance of LTP in the CA1 of mouse hippocampus (Fig. 2A). In accord with our previous studies, CM from sister cultures of untransfected CHO cells had no effect on LTP (Fig. 2A). Importantly, medium from 7PA2 cells conditioned with either RS-0406 or RS-0466 produced no inhibition of LTP (Fig. 2, compare A,B). Indeed, the potentiation observed with the CM of cells treated with either of the hydroxyanaline compounds did not differ significantly from that obtained in the presence of plain CHO-CM at 60 min post-HFS (CHO, 232.9 ± 17.3%; RS-0406, 196.7 ± 11.3%; RS-0466, 192.1 ± 19.4%; mean ± SEM percentage of baseline; n = 3 and n = 4; p > 0.05), compared with the pre-HFS baseline (Fig. 2D).
Figure 2.
Conditioned medium from 7PA2 cells treated with RS-0406 or RS-0466 do not alter hippocampal LTP. A, Perfusion of hippocampal slices with 7PA2 CM (blue), but not with CHO-CM (black), blocked LTP of excitatory synaptic transmission in the CA1 area [131.3 ± 8.3% at 60 min after HFS (indicated by arrows); mean ± SEM percentage of baseline; n = 6; p > 0.05 compared with pre-HFS baseline]. Insets show typical EPSPs ∼5 min pre- (1) and ∼60 min post-HFS (2). Calibration: 10 ms, 1 mV. Asterisk denotes addition of test media. B, Perfusion of hippocampal slices with CM from 7PA2 cells conditioned in the presence of RS-0406 (red) or RS-0466 (green) resulted in no inhibition of LTP (196.7 ± 11.3% at 60 min post-HFS, n = 7, p > 0.05; 196.7 ± 19.4% at 60 min post-HFS, n = 7, p > 0.05, respectively). Calibration: 25 ms, 2 mV. C, Perfusion of hippocampal slices with 7PA2 CM spiked acutely with RS-0406 (red open circles) or RS-0466 (green open circles) did not reverse the inhibition of LTP (113.9 ± 3.6% at 60 min post-HFS, n = 5, p > 0.05; 111.1 ± 8.4% at 60 min post-HFS, n = 7, p > 0.05, respectively), Calibration: 25 ms, 2 mV. D, Magnitude of LTP at 60 min post-HFS (percentage of baseline ± SEM). Treatments and the number of slices examined per treatment are indicated beneath each histogram. All treatments refer to the use of 7PA2 CM unless otherwise indicated. The term post indicates that compound was added to medium after it had been conditioned by cells, as opposed to addition before conditioning. Medium conditioned by 7PA2 cells in the presence of either RS-0406 or RS-0466 facilitated normal LTP, whereas addition of either compound after conditioning did not prevent the oligomer-mediated block of LTP. Error bars represent SEM.
IP/wblot analyses of the CM used for the electrophysiology experiments shown in Figure 2B were almost identical to those shown in Figure 1B (i.e., they contained abundant monomer but very little oligomers) (data not shown). To determine whether this recovery of LTP was caused by a reduction in the oligomer content of the 7PA2 CM (Fig. 1B) or rather to a direct action of the hydroxyanaline derivatives on hippocampal slices, we performed a number of control experiments. CM from 7PA2 cells was directly spiked with either RS-0406 or RS-0466 (at the same concentrations used in Fig. 1) and then tested immediately on hippocampal slices. Such medium still contained abundant oligomers (Fig. 1E) and still caused a block of LTP indistinguishable from that induced by untreated 7PA2 CM (Fig. 2D, compare A,C). Moreover, addition of RS-0406 and RS-0466 to 7PA2 CM caused no change in basal synaptic activity (RS-0406, 108.1 ± 9.5%; RS-0466, 98.4 ± 6.5% at 60 min post-HFS; mean ± SEM percentage of baseline; n = 3 and n = 4; p > 0.05) compared with the pre-HFS baseline (Fig. 2D). Also, we observed normal induction and maintenance of LTP in the presence of CHO-CM spiked with either compound (RS-0406, 200.4 ± 9.4%; RS-0466, 207.1 ± 24.6% at 60 min post-HFS; mean ± SEM percentage of baseline; n = 4 and n = 3; p < 0.05) compared with the pre-HFS baseline (Fig. 2D). Together, these negative controls strongly suggest that treatment of 7PA2 cells with RS-0406 and RS-0466 allows the maintenance of normal LTP because of their ability to decrease the oligomer content of 7PA2 CM. These results add to the burgeoning evidence that Aβ oligomers, not Aβ monomers, can induce synaptic dysfunction that results in altered LTP.
Size exclusion fractionation of secreted Aβ monomers and oligomers
The above findings demonstrate that the specific reduction of oligomer levels (Fig. 1) results in a loss of the ability of 7PA2 CM to inhibit maintenance of LTP (Fig. 2). This result is in accord with our previous demonstration that pretreatment of 7PA2 CM with insulin-degrading enzyme, which can degrade Aβ monomers but not oligomers, did not prevent the inhibition of LTP (Walsh et al., 2002a). Although these distinct approaches strongly support the concept that low-n oligomers, but not monomers, of Aβ are synaptotoxic, we sought to provide additional evidence for this hypothesis. To this end, we developed a method to fractionate 7PA2 CM under nondenaturing conditions to separate Aβ monomers from oligomers. SEC fractionates on the basis of molecular weight, is performed using nondenaturing and nondisaggregating buffers, and has proved useful in the isolation and characterization of intermediates of synthetic Aβ fibrillogenesis (Walsh et al., 1997, 1999; Hartley et al., 1999; Ye et al., 2004). Using a Superdex 75 column and a volatile, mildly alkaline buffer, we achieved the complete separation of Aβ monomer from Aβ oligomers, as judged by Western blotting of each SEC fraction (Fig. 3). Cell-derived Aβ monomer and oligomers are detected by a variety of antibodies to the mid, N-, and C-terminal regions of Aβ (Podlisny et al., 1995; Walsh et al., 2000). Therefore, to confirm the authenticity of the various bands detected by Western blotting of SEC-fractionated 7PA2 CM, we used four anti-Aβ monoclonal antibodies directed to different regions of Aβ.
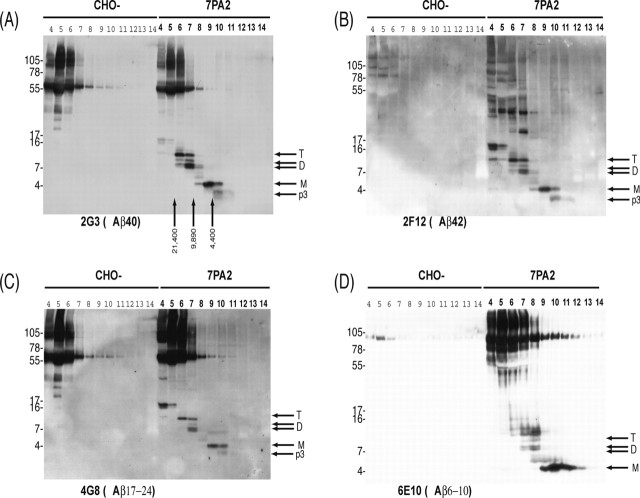
Figure 3.
Size exclusion fractionated oligomers are detected by antibodies to the mid region, N terminus, and C terminus of Aβ. Conditioned media from CHO and 7PA2 cells were concentrated ∼10-fold and then chromatographed on a Superdex 75 column, and 1 ml fractions were collected, lyophilized, and Western blotted. Lanes are labeled according to elution volume, and the elution of dextran standards is indicated by vertical arrows. Antibodies to the mid region (C; 4G8), N terminus (D; 6E10), and C terminus (A, 2G3; B, 21F12) of Aβ revealed the resolution of Aβ monomers (M), dimers (D), and trimers (T). The specificity of these bands is confirmed by the fact that they are only detected in fractions of 7PA2 CM (fraction numbers in bold type) and not in fractions of CHO-CM. Samples shown in A and B were from the same fractionation, and those shown in C and D were from separate fractionations. In each case, the blots shown are representative of at least three different experiments.
The p3 peptide (the proteolyic product of α- and γ-secretase processing of APP), Aβ monomer, and Aβ oligomers were all detected in SEC fractions immunoblotted with 2G3 (an antibody that specifically detects Aβ peptides with C termini ending at residue 40). SEC separates molecules such that larger molecules elute in earlier fractions and smaller ones in later fractions. For instance, the ∼3 kDa p3 peptide eluted in fractions 11 and 10, whereas Aβ monomer was detected in fractions 10, 9, and 8. As the amount of Aβ monomer fell, the amounts of a ∼5 kDa Aβ species [a conformer of the Aβ monomer (Walsh et al., 2002a)] and the dimer increased (Fig. 3A, fraction 7). Dimers and trimers were most abundant in fractions 7 and 6, respectively (Fig. 3A). In fractions 5 and 4, a faint putative tetramer band was detected (Fig. 3A). No bands migrating between 3 and 17 kDa were detected by 2G3 in CM from CHO control cells.
Western blotting with 21F12 (an antibody that specifically recognizes Aβ peptides with C termini ending at residue 42) produced a closely similar pattern of staining of p3, Aβ monomer, 5 kDa Aβ conformer, dimer, and trimer to that revealed by 2G3 (compare Fig. 3A,B). However, there were two notable differences using 21F12: (1) the putative tetramer band (∼16-17 kDa) was much more intensely labeled than it was by 2G3; and (2) a ladder of bands between ∼30 and ∼75 kDa was detected that was not present in the corresponding fractions of the CHO-CM. The authenticity of the ∼30-75 kDa bands is questionable, because they were not detected by any of the other anti-Aβ antibodies tested (Fig. 3A-D).
Like 2G3 and 21F12, 6E10, an antibody to residues 6-10 of Aβ, detected Aβ monomer, 5 kDa Aβ conformer, dimer, trimer, and a faint tetramer band but did not detect p3, as would be expected from the absence of the 6E10 epitope in p3. 6E10 also detected soluble APP, which can be seen in the high-molecularweight range of the gel. Monoclonal antibody 4G8, an antibody to the mid region of Aβ, produced a pattern similar to that of 2G3. It is noteworthy that the detection of the putative tetramer was more variable than that of the monomer, dimer, and trimer bands, which were detected in all blots and by all antibodies tested in >50 independent experiments. Moreover, the elution pattern was highly similar, but much weaker, when unconcentrated CM was used for SEC.
Size exclusion chromatography confirms that compounds RS-0406 and RS-0466 inhibit oligomer formation and that oligomers are responsible for the block of LTP
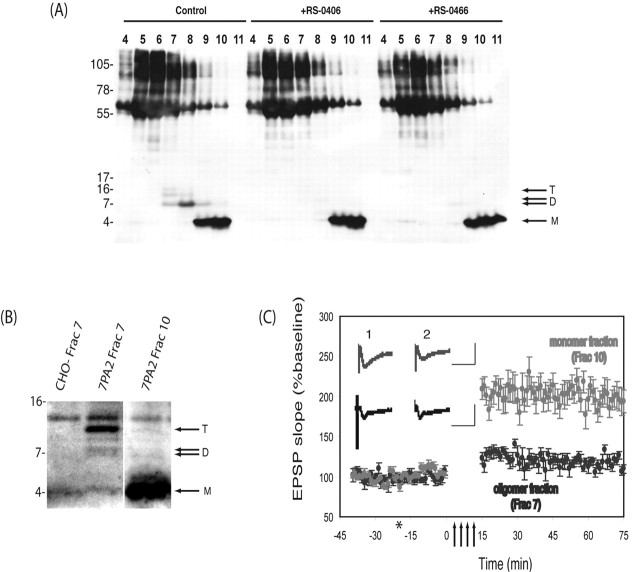
CM from 7PA2 cells grown in the presence of the two compounds shown to inhibit oligomer formation (Fig. 1B) was subjected to SEC, and the fractions were analyzed by Western blotting. The CM of 7PA2 cells not exposed to RS-0406 or RS-0466 produced a pattern highly similar to that shown previously (compare the first 7 lanes of Fig. 4A with lanes 4-10 of the 7PA2 samples in Figs. 3A-D); again SEC fractions 9 and 10 contained Aβ monomer, fraction 8 contained both dimer and trimer, and fraction 7 contained dimer, trimer, and tetramer. Treatment of the cells with either RS-0406 or RS-0466 resulted in a near-complete loss of oligomers without significantly changing Aβ monomer levels. The fact that the SEC-isolated oligomer species are specifically removed by the action of these compounds supports the authenticity of these fractionated species as Aβ oligomers.
Figure 4.
SEC-isolated Aβ oligomers, but not monomers, inhibit LTP. A, 7PA2 cells were allowed to condition serum-free medium in the presence or absence of RS-0406 or RS-0466 for 15 h, and the CM was concentrated as described. The concentrated CM was subjected to SEC, and fractions were analyzed by Western blotting using a combination of 2G3 and 21F12. Aβ monomers (M), dimers (D), and trimers (T) are indicated by arrows. B, SEC fractions containing monomeric or oligomeric Aβ were lyophilized and reconstituted in 10 ml of ACSF, and an aliquot (1.5 ml) was analyzed by IP/Western blot. C, Perfusion of hippocampal slices with oligomeric Aβ (fraction 7) inhibited LTP (115.8 ± 10.0% at 60 min post-HFS; mean ± SEM percentage of baseline; n = 6; p > 0.05 compared with pre-HFS baseline), whereas perfusion with monomeric Aβ (fraction 9) did not alter LTP (227.1 ± 22.6% at 60 min post-HFS; mean ± SEM percentage of baseline; n = 6; p > 0.05 compared with pre-HFS baseline). Insets show typical EPSPs ∼5 min pre- (1) and ∼60 min post-HFS (2). Calibration: 25 ms, 2 mV. Arrows signify the period of HFS, and the asterisk indicates addition of test media.
Because we showed above that CM from 7PA2 cells grown in the presence of either RS-0406 or RS-0466 contained little or no oligomers (Figs. 1B, C, 4A) and that this CM did not inhibit LTP, we sought to confirm the electrophysiological effects of the SEC-isolated Aβ monomer and oligomer fractions (Fig. 4B). As expected from the previous data, the oligomer-containing SEC fractions caused a dramatic block of LTP (Fig. 4C), whereas monomer-containing SEC fractions caused no such block (oligomer, 109.6 ± 4.9% vs monomer, 194.2 ± 14.1% at 60 min post-HFS; mean ± SEM percentage of baseline; n = 3 and n = 4; p < 0.05) compared with the pre-HFS baseline (Fig. 4C). This was not a result of differences in Aβ concentration, because the total amount of Aβ in the monomer-containing fraction far exceeded that in the oligomer-containing fraction of the same SEC run (Fig. 4B). Importantly, the Western analysis shown in Figure 4B demonstrates that the lyophilization and subsequent dilution of SEC-isolated Aβ species does not alter the aggregation state of the fractionated peptides.
Discussion
Aβ fibrillogenesis is a principal target for therapeutic intervention in Alzheimer's disease and related human β-amyloidoses (Findeis, 2002). However, in view of the growing literature suggesting a potentially key pathogenic role for soluble oligomers of Aβ (Rochet and Lansbury, 2000; Hardy and Selkoe, 2002; Cohen and Kelly, 2003; Walsh et al., 2003; Klein et al., 2004), it is important that inhibitors of fibrillogenesis act to inhibit the initial stages of oligomerization. If inhibition of fibril formation were to lead to an accumulation of pathologically active prefibrillar assemblies such as low-n oligomers, this strategy might lead to enhanced rather than decreased neurotoxicity.
Here, we show that certain small-molecule inhibitors of synthetic Aβ fibrillogenesis can also block formation of cell-derived, secreted oligomers of Aβ and that inhibition of these natural oligomers fully prevents the well characterized block of LTP that is mediated by Aβ. In contrast, two peptidic compounds, OR1 and OR2, both of which are more potent inhibitors of synthetic fibrillogenesis than RS-0406 or RS-0466, do not alter formation of the cell-derived oligomers. The reason for this failure is unclear but could involve one or more of three factors: (1) the inability of OR1 and OR2 to prevent initial oligomerization (i.e., they may inhibit formation of large oligomers and polymers but do not alter the formation of soluble, low-n oligomers), (2) the failure of these compounds to access the appropriate subcellular compartment in which oligomerization occurs, or (3) a lack of stability of these compounds in cell culture. We have shown previously that Aβ oligomerization (i.e., dimer formation) can begin intracellularly (Walsh et al., 2000, 2002a), and it is not clear that OR1 and OR2 can penetrate the cell. This lack of efficacy of OR1 and OR2 in the face of successful inhibition by RS-0406 and RS-0466 highlights the utility of our cell-culture paradigm as a useful and important secondary screen for compounds designed to inhibit early steps in Aβ oligomerization under biologically relevant conditions.
We have demonstrated previously that Aβ oligomer-containing culture medium can consistently inhibit the maintenance of hippocampal LTP (Walsh et al., 2002a; Wang et al., 2004). Here, we use size fractionation of Aβ to show that this effect on synaptic plasticity can be attributed to low-n oligomers, principally dimers and trimers. In a previous study, we used insulin degrading enzyme (IDE), an enzyme that specifically degrades Aβ monomer, but not oligomers, to deplete 7PA2 CM of Aβ monomer and concluded that the observed block of LTP was caused by Aβ oligomers (Walsh et al., 2002a). An important advantage of SEC over the use of selective degradation by IDE is the ability of SEC to resolve Aβ oligomers from both Aβ monomer and p3, whereas IDE degrades Aβ monomer but leaves p3 unaffected. Thus, our SEC paradigm allows us to unambiguously attribute the Aβ-mediated block of LTP to low-n oligomers in the absence of fibrillar and monomeric Aβ and p3. Furthermore, elution of Aβ oligomers in the included volume of the column under nondenaturing conditions provides additional evidence that the Aβ species that migrate on denaturing PAGE at low molecular weight are not electrophoresis artifacts or breakdown products of larger assemblies such as protofibrils.
Using SEC and Western blotting, we confirmed that RS-0406 and RS-0466 block natural oligomer formation and that these compounds prevent the Aβ-mediated block of LTP specifically by inhibiting oligomer formation. In the case of RS-0406, these results are in accord with a previous study that found RS-0406 to effectively inhibit synthetic Aβ aggregation and thus relieve the Aβ-mediated block of LTP (Nakagami et al., 2002a). However, our results with RS-0466 are slightly different from previous work (Nakagami et al., 2002a). Like Nakagami et al., we found that RS-0406, but not RS-0466, inhibited fibrillogenesis of synthetic Aβ1-42 (Nakagami et al., 2002b), whereas in our cell-culture experiments, both compounds inhibited the formation of natural Aβ oligomers. Furthermore, these compounds were only effective in preventing the Aβ-mediated block of LTP under conditions in which they prevented new oligomer formation by intact cells and not when added to CM already containing oligomers (Fig. 2A,B).
In the experiments conducted by Nakagami et al. (2002a,b), synthetic Aβ1-42 was incubated at 100 μm for 1-2 d, diluted to 1 μm in ACSF with or without RS-0406 or RS-0466, and incubated on hippocampal slices for an additional 5 h. Slices were then perfused with fresh ACSF and used to record LTP. It is not clear how RS-0406 and RS-0466 prevented the block of LTP mediated by Aβ1-42: did they bind to or destabilize synthetic Aβ oligomers, or did they act directly on the neurons? Although these compounds did not appear to destabilize the natural, SDS-stable oligomers once they were formed, we speculate that oligomers formed by synthetic Aβ are less stable than these natural oligomers and that RS-0406 and RS-0466 may be able to destabilize the former. In this regard, it is noteworthy that we previously found natural cell-derived Aβ to be at least two orders of magnitude more potent at inhibiting LTP than was synthetic Aβ1-42 applied under otherwise identical conditions (Wang et al., 2004). It is clear that both RS-0406 and RS-0466 are capable of inhibiting oligomerization of cell-derived Aβ and preventing the Aβ-mediated block of LTP. Thus, our work establishes the utility of a novel Aβ-secreting cell culture system for screening and optimizing compounds that inhibit the earliest stages of natural Aβ assembly without inducing cellular injury.
Footnotes
This work was supported by National Institutes of Health Grants AG05134 (D.J.S.) and AG19770 (D.M.H.), by the Foundation for Neurologic Diseases, and by Wellcome Trust Grant 067660 (D.M.W.). We thank Dr. Yasuhiro Nakagami (Sankyo, Tokyo, Japan) and Dr. Russell Hagan (BTG International, London, UK) for the gift of RS-0406 and RS-0466 and Dr. Vicki Betts for expert technical assistance.
Correspondence should be addressed to Dennis J. Selkoe, Center for Neurologic Diseases, Harvard Institutes of Medicine, 77 Avenue Louis Pasteur, Room 730, Boston, MA 02115-5716. E-mail: dselkoe@rics.bwh.harvard.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/252455-08$15.00/0
D.M.W. and M.T. contributed equally to this work.
References
- Cohen FE, Kelly JW (2003) Therapeutic approaches to protein-misfolding diseases. Nature 426: 905-909. [DOI] [PubMed] [Google Scholar]
- Davies CA, Mann DM, Sumpter PQ, Yates PO (1987) A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci 78: 151-164. [DOI] [PubMed] [Google Scholar]
- Findeis MA (2002) Peptide inhibitors of beta amyloid aggregation. Curr Top Med Chem 2: 417-423. [DOI] [PubMed] [Google Scholar]
- Haass C, Schlossmacher M, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski B, Lieberburg I, Koo EH, Schenk D, Teplow D, Selkoe D (1992) Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359: 322-325. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353-356. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ (1999) Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci 19: 8876-8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L (1999) Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci USA 96: 3228-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida N, Hartmann T, Pantel J, Schroder J, Zerfass R, Forstl H, Sandbrink R, Masters CL, Beyreuther K (1996) Analysis of heterogeneous A4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay. J Biol Chem 271: 22908-22914. [DOI] [PubMed] [Google Scholar]
- Klein WL, Stine Jr WB, Teplow DB (2004) Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer's disease. Neurobiol Aging 25: 569-580. [DOI] [PubMed] [Google Scholar]
- Koo EH, Squazzo S (1994) Evidence that production and release of amyloid β-protein involves the endocytic pathway. J Biol Chem 269: 17386-17389. [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL (1998) Diffusible, nonfribrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95: 6448-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J (1999) Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol 155: 853-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL (1999) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol 46: 860-866. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Ihara Y (1998) The presence of amyloid β-protein in the detergent-insoluble membrane compartment of human neuroblastoma cells. Biochemistry 37: 15247-15253. [DOI] [PubMed] [Google Scholar]
- Moyer Jr JR, Brown TH (1998) Methods for whole-cell recording from visually preselected neurons of perirhinal cortex in brain slices from young and aging rats. J Neurosci Methods 86: 35-54. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L (2000) High-level neuronal expression of a β1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 20: 4050-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami Y, Nishimura S, Murasugi T, Kaneko I, Meguro M, Marumoto S, Kogen H, Koyama K, Oda T (2002a) A novel beta-sheet breaker, RS-0406, reverses amyloid beta-induced cytotoxicity and impairment of long-term potentiation in vitro. Br J Pharmacol 137: 676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami Y, Nishimura S, Murasugi T, Kubo T, Kaneko I, Meguro M, Marumoto S, Kogen H, Koyama K, Oda T (2002b) A novel compound RS-0466 reverses beta-amyloid-induced cytotoxicity through the Akt signaling pathway in vitro. Eur J Pharmacol 457: 11-17. [DOI] [PubMed] [Google Scholar]
- Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydel RE, Teplow DB, Selkoe DJ (1995) Aggregation of secreted amyloid β-protein into SDS-stable oligomers in cell culture. J Biol Chem 270: 9564-9570. [DOI] [PubMed] [Google Scholar]
- Rochet JC, Lansbury Jr PT (2000) Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol 10: 60-68. [DOI] [PubMed] [Google Scholar]
- Roher AE, Chaney MO, Kuo Y-M, Webster SD, Stine WB, Haverkamp LJ, Woods AS, Cotter RJ, Tuohy JM, Krafft GA, Bonnell BS, Emmerling MR (1996) Morphology and toxicity of Aβ-(1-42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer's disease. J Biol Chem 271: 20631-20635. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2002) Alzheimer's disease is a synaptic failure. Science 298: 789-791. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM, Moskal JR (1987) Inhibition of the production and maintenance of long-term potentiation in rat hippocampal slices by a monoclonal antibody. Proc Natl Acad Sci USA 84: 1684-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Lomakin A, Benedek GB, Maggio JE, Condron MM, Teplow DB (1997) Amyloid β-protein fibrillogenesis: detection of a protofibrillar intermediate. J Biol Chem 272: 22364-22374. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB (1999) Amyloid β-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem 274: 25945-25952. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ (2000) Detection of intracellular oligomers of amyloid β-protein in cells derived from human brain. Biochemistry 39: 10831-10839. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva J, William K. Cullen W, Anwyl R, Wolfe M, Rowan M, Selkoe D (2002a) Naturally secreted oligomers of the Alzheimer amyloid β-protein potently inhibit hippocampal long-term potentiation in vivo Nature 416: 535-539. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ (2002b) Amyloid-β oligomers: their production, toxicity and therapeutic inhibition. Biochem Soc Trans 30: 552-557. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Hartley DM, Selkoe DJ (2003) The many faces of Aβ: structures and activity. Curr Med Chem Immunol Endocrinol Metab Agents 3: 277-291. [Google Scholar]
- Wang J, Dickson DW, Trojanowski JQ, Lee VM (1999) The levels of soluble versus insoluble brain Abeta distinguish Alzheimer's disease from normal and pathologic aging. Exp Neurol 158: 328-337. [DOI] [PubMed] [Google Scholar]
- Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R (2004) Block of long-term potentiation by naturally secreted and synthetic amyloid β-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci 24: 3370-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Zhang J, Kholodenko D, Citron M, Podlisny MB, Teplow DB, Haass C, Seubert P, Koo EH, Selkoe DJ (1997) Enhanced production and oligomerization of the 42-residue amyloid β-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J Biol Chem 272: 7977-7982. [DOI] [PubMed] [Google Scholar]
- Ye C, Walsh DM, Selkoe DJ, Hartley DM (2004) Amyloid β-protein induced electrophysiological changes are dependent on aggregation state: N-methyl-d-aspartate (NMDA) versus non-NMDA receptor/channel activation. Neurosci Lett 366: 320-325. [DOI] [PubMed] [Google Scholar]