Figure 1.
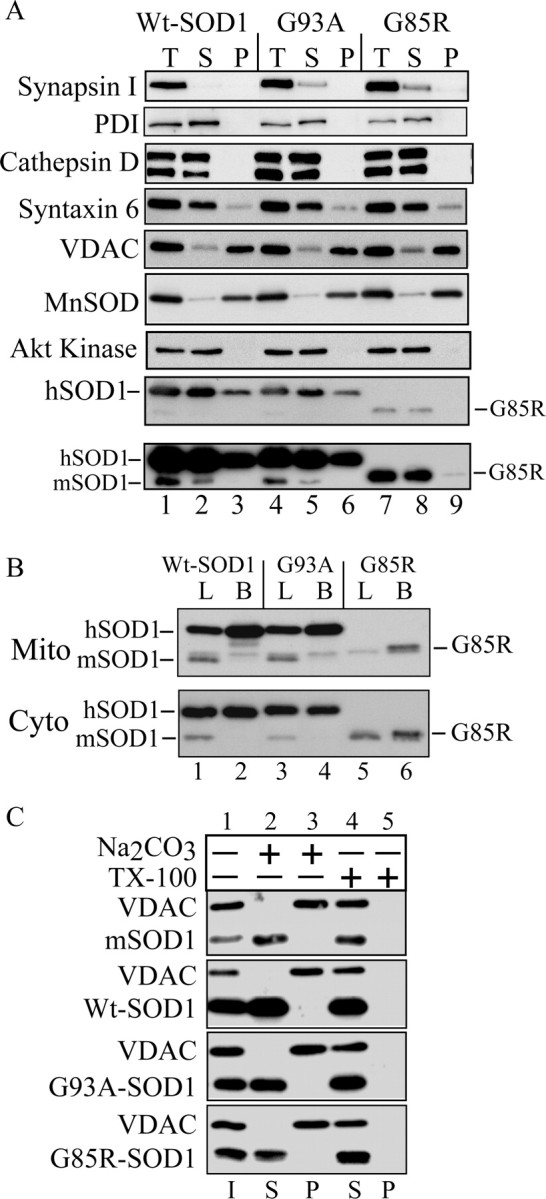
SOD1 in purified mitochondria from fALS transgenic mice. A, Immunoblotting of volume equivalents of postnuclear supernatant (T), postmitochondrial supernatant (S), and purified mitochondria (P). Mitochondrial content and structural integrity were analyzed using antibodies against the mitochondrial markers MnSOD and VDAC. Contamination was assessed with antibodies against synapsin I (synaptic vesicles), PDI (endoplasmic reticulum) and cathepsin D (lysosomes), Akt kinase (cytosol), and syntaxin 6 (trans-Golgi network). SOD1 was detected with antibodies that react with both the mouse SOD1 (mSOD1) and the transgenic hSOD1. The position of the faster-migrating G85R mutant hSOD1 is indicated. The bottom panel is a prolonged exposure of the above SOD1 blot to detect small amounts of G85R hSOD1 in the mitochondrial pellet. B, Comparison of the relative SOD1 content in purified mitochondria (Mito) and cytosol (Cyto) from brain and liver of hSOD1 transgenic mice. B, Brain; L, liver. C, Alkali treatment of mitochondrial membranes. Purified brain mitochondria were incubated in the presence of 0.1 m sodium carbonate, pH ∼11.5, or buffer H plus 0.5% TX-100. After centrifugation, supernatants (S) and pellets (P) were analyzed by SDS-PAGE and immunoblotting for SOD1 and the mitochondrial outer membrane protein VDAC. I, Input.
