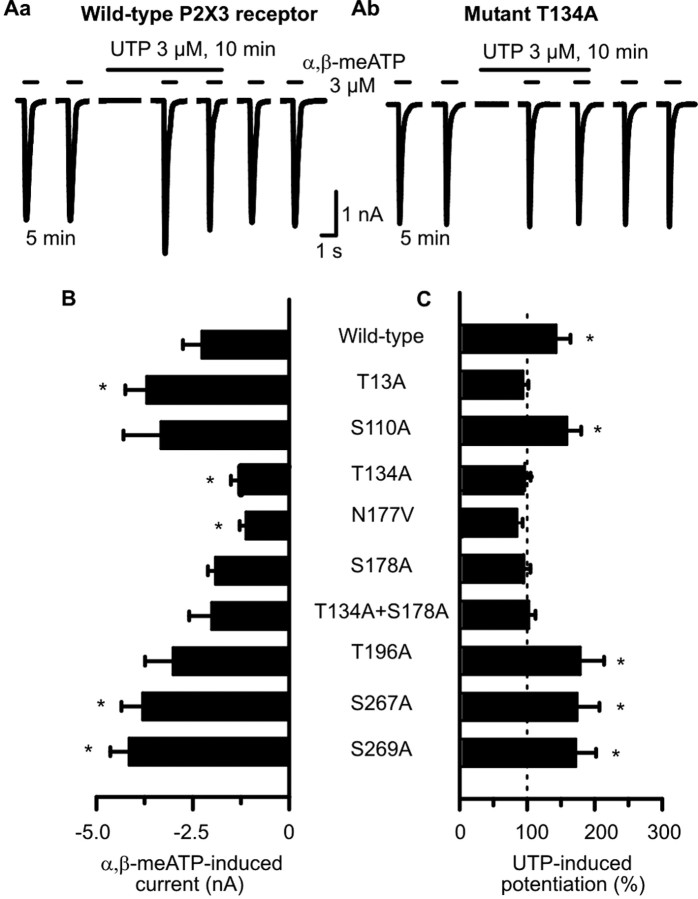
Figure 4.
Mutation of Ser and Thr residues in consensus PKC phosphorylation sites of hP2X3 receptors alters the sensitivity of these receptors toward α,β-meATP in HEK293-P2X3 cells. A transient transfection protocol was used. The experimental procedures were similar to those described for Figure 1 B. Patch pipettes filled with GDP-β-S (300 μm)-containing solution were used. Superfusion with UTP (300 μm) started 5 min before the third application of α,β-meATP. A, Original tracings show that the α,β-meATP-induced currents were potentiated by UTP (3 μm) in the wild-type hP2X3 receptor (Aa) but not in its mutant T134A (Ab). B, Amplitudes of α,β-meATP (3 μm) currents recorded from HEK293 cells transfected with the wild-type hP2X3 receptor or its mutants. Mean ± SEM of 7-14 experiments. C, Potentiation of α,β-meATP (3 μm) current amplitudes by UTP (3 μm) evoked at wild-type hP2X3 receptors but not at some of their mutants. Mean ± SEM of seven to eight experiments similar to those shown in Aa and Ab. Substitution of Ser and Thr residues localized within the extracellular consensus PKC sites near transmembrane domain 2 with Ala (T196A, S269A) failed to interfere with the facilitatory effect of UTP. In contrast, identical mutations in the Ser and Thr residues localized within the two extracellular consensus PKC sites near transmembrane domain 1 (T134A, S178A) abolished the action of UTP. *p < 0.05, statistically significant difference from the wild-type receptor (B) or from 100% (C). The comparison of A and B indicates that all mutants react to agonist application with stable current responses, and there is no correlation between the magnitude of these currents and the ability of UTP to facilitate them.