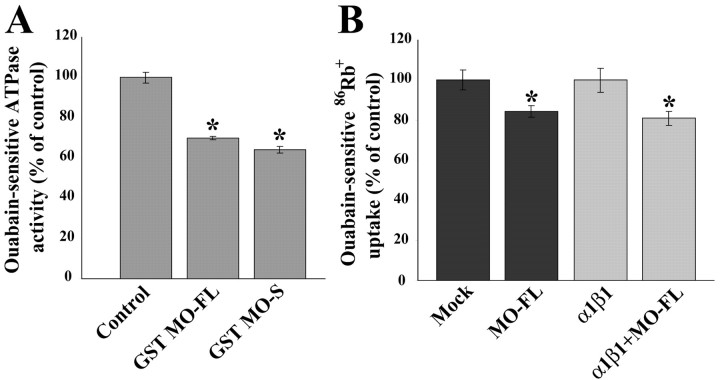
Figure 9.
GST MONaKA inhibits Na,K-ATPase activity in vitro and 86Rb+ uptake in intact cells. A, The production of inorganic phosphate from ATP by a partially purified Na,K-ATPase preparation was measured by a colorimetric assay. The ouabain-sensitive portion of the total ATPase activity was taken as the basal Na,K-ATPase activity (1.2 μmol Pi/mgprotein/min). This activity is inhibited by both short (S) and full-length (FL) GST-MONaKA (MO) but not by GST alone or GST-Drosophila Slob (data not shown) (*p < 0.05, significantly different from control). Similar results were obtained with Na,K-ATPase from purified cultured astrocytes (data not shown). B, tsA201 cells were transfected with vector (Mock) with or without full-length MONaKA (left two lanes) or with α1β1 Na,K-ATPase subunits with or without full-length MONaKA (right two lanes). The ouabain-sensitive portion of total 86Rb+ uptake was taken as that attributable to the Na,K-ATPase. Identical results were obtained with the short version of MONaKA (data not shown). Each data point is the mean ± SE of three experiments (*p < 0.05, significantly different from transfection without MONaKA). Both endogenous and α1β1-mediated 86Rb+ uptake are inhibited by MONaKA.