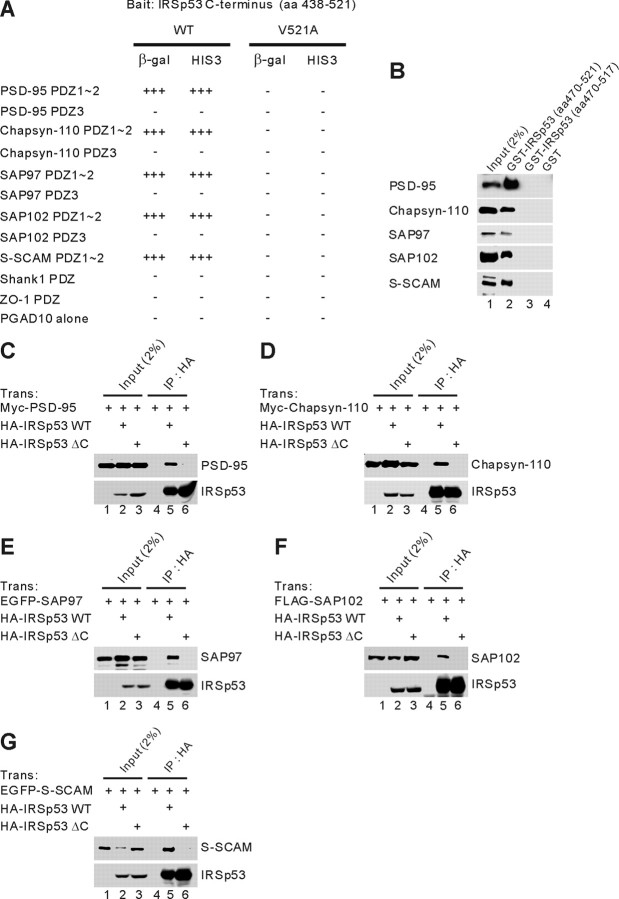
Figure 1.
IRSp53 interacts with all known PSD-95 family proteins and S-SCAM in yeast two-hybrid, GST pull-down, and coimmunoprecipitation assays. A, Interaction of IRSp53 with PSD-95 family proteins and S-SCAM in the yeast two-hybrid assay. PDZ domains from PSD-95 family proteins, S-SCAM, and other PDZ proteins in pGAD10 (prey vector) were tested for binding to IRSp53 (aa 438-521; wild type and the V521A mutant, in which the last Val residue was changed to Ala) in pBHA (bait vector) in the yeast two-hybrid assay. β-Galactosidase (β-gal) activity: +++, <45 min; ++, 45-90 min; +, 90-240 min; -, no significant β-gal activity. HIS3 activity: +++, >60%; ++, 30-60%; +, 10-30%; -, no significant growth. B, Pull down of PSD-95 family proteins and S-SCAM with GST-IRSp53 fusion proteins. GST fusion proteins of IRSp53 (aa 470-521 and aa 470-517, which lacks the last 4 residues) and GST alone were used to bring down the indicated proteins expressed in human embryonic kidney 293T (HEK293T) cells. The precipitates were analyzed by immunoblotting with the indicated antibodies. C, Lysates of HEK293T cells doubly transfected with Myc-PSD-95 plus HA-IRSp53WT or a mutant lacking the last four residues (IRSp53ΔC), or singly with Myc-PSD-95 were immunoprecipitated with HA antibodies and immunoblotted with HA (for IRSp53) and Myc (for PSD-95) antibodies. Trans, Transfection; IP, immunoprecipitation. HA, Myc, FLAG, and EGFP represent epitope tags. D-G, Chapsyn-110 (D), SAP97 (E), SAP102 (F), and S-SCAM (G) were similarly analyzed for coimmunoprecipitation with IRSp53.