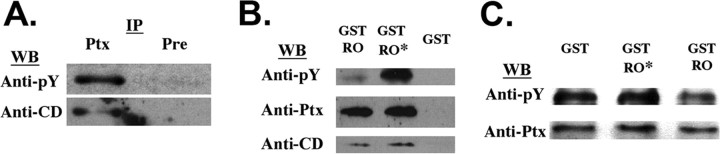
Figure 4.
The 41 kDa NPCD protein is a PTPRO substrate. A, Immunoprecipitations were performed using the Ptx antibody (Ptx) or preimmune serum (Pre) from adult brain. The precipitates were probed with anti-phosphotyrosine (anti-pY) and were then stripped and reprobed with the CD antibody. An NPCD band at 41 kDa that is precipitated by the Ptx antibody and recognized by the CD antibody is tyrosine phosphorylated in vivo. B, GST pulldowns were performed from mouse brain lysates using GST-PTPRO (GST-RO), a catalytically inactive mutant of GST-PTPRO (GST-RO*), or GST alone. Precipitates were probed successively with a phosphotyrosine antibody, the Ptx antibody, and the CD antibody. Similar amounts of the 41 kDa NPCD protein were specifically associated with either the wild-type or inactive form of PTPRO. However, phosphotyrosine levels were much lower when the wild-type PTPRO was used, indicating that the 41 kDa NPCD isoform is a PTPRO substrate. C, Native 41 kDa NPCD protein was isolated from brain by immunoprecipitation using the Ptx antibody and was then incubated at 37°C for 1 h with 5 μg of purified GST, catalytically inactive GST-PTPRO, or GST-PTPRO. After incubation, NPCD was immunoprecipitated and probed successively with a phophotyrosine antibody and the Ptx antibody. NPCD is directly dephosphorylated by GST-PTPRO but not by the inactive mutant. WB, Western blot; IP, immunoprecipitation.