Abstract
Calcium plays a crucial role as a ubiquitous second messenger and has a key influence in many forms of synaptic plasticity in neurons. The spatiotemporal properties of dendritic Ca2+ signals in hippocampal interneurons are relatively unexplored. Here we use two-photon calcium imaging and whole-cell recordings to study properties of dendritic Ca2+ signals mediated by different glutamate receptors and their regulation by synaptic activity in oriens/alveus (O/A) interneurons of rat hippocampus. We demonstrate that O/A interneurons express Ca2+-permeable AMPA receptors (CP-AMPARs) providing fast Ca2+ signals. O/A cells can also coexpress CP-AMPARs, Ca2+-impermeable AMPARs (CI-AMPARs), and group I/II metabotropic glutamate receptors (mGluRs) (including mGluR1a), in the same cell. CI-AMPARs are often associated with mGluRs, resulting in longer-lasting Ca2+ signals than CP-AMPAR-mediated responses. Finally, CP-AMPAR- and mGluR-mediated Ca2+ signals demonstrate distinct voltage dependence and are differentially regulated by presynaptic and postsynaptic activity: weak synaptic stimulation produces Ca2+ signals mediated by CP-AMPARs, whereas stronger stimulation, or weak stimulation coupled with postsynaptic depolarization, recruits Ca2+ signals mediated by mGluRs. Our results suggest that differential activation of specific glutamate receptor-mediated Ca2+ signals within spatially restricted dendritic microdomains may serve distinct signaling functions and endow oriens/alveus interneurons with multiple forms of Ca2+-mediated synaptic plasticity. Specific activation of mGluR-mediated Ca2+ signals by coincident presynaptic and postsynaptic activity fulfills the conditions for Hebbian pairing and likely underlies their important role in long-term potentiation induction at O/A interneuron synapses.
Keywords: Ca2+-permeable AMPA receptor, group I mGluR, Ca2+ microdomains, synaptic Ca2+ signals, two-photon confocal imaging, dendritic microdomains
Introduction
The cellular basis for information processing and storage in the brain is provided by long-lasting, activity-dependent changes in synaptic efficacy, such as long-term potentiation (LTP). Hippocampal CA1 interneurons in stratum oriens/alveus (O/A) demonstrate a Hebbian form of LTP that depends on the activation of the metabotropic glutamate receptor (mGluR) 1a subtype of mGluRs, occurs at synapses containing Ca2+-permeable (CP)-AMPARs, and requires postsynaptic Ca2+ rises (Perez et al., 2001; Lapointe et al., 2004). This form of LTP does not depend on the activation of NMDA receptors (NMDARs).
AMPA receptors of interneurons, in contrast to those of pyramidal cells, are heteromers often characterized by an absence of GluR2 subunits that renders them Ca2+ permeable (Jonas and Burnashev, 1995). It is well established that CP-AMPARs participate in synaptic transmission in some inhibitory interneurons (McBain and Dingledine, 1993; Isa et al., 1996). Moreover, they endow interneuron synapses with short- and long-term plasticity (Mahanty and Sah, 1998; Rozov et al., 1998; Laezza et al., 1999; Rozov and Burnashev, 1999; Toth et al., 2000; Lei and McBain, 2002, 2004). In neocortical aspiny interneurons, CP-AMPARs mediate a spine-free mechanism of input-specific calcium compartmentalization (Goldberg et al., 2003c); however, in hippocampal O/A interneurons, the spatiotemporal properties of Ca2+ signals mediated by CP-AMPARs and their contribution to synaptic activity and LTP induction are presently unknown.
Group I mGluRs (specifically mGluR1a) are highly expressed in O/A interneurons (Masu et al., 1991; Baude et al., 1993; Lujan et al., 1996). It has been shown that these receptors can be activated synaptically in O/A cells (Huang et al., 2004). Moreover, group I mGluR agonists produce intracellular Ca2+ rises in these cells (Carmant et al., 1997; Woodhall et al., 1999; Gee and Lacaille, 2004) that arise from a coupling of Ca2+ entry via voltage-gated calcium channels (VGCCs) and intracellular Ca2+ release (Woodhall et al., 1999); however, the properties and spatial localization of group I mGluR-mediated Ca2+ signals in interneuron dendrites are unknown. Because LTP in O/A interneurons requires postsynaptic Ca2+ rises, the relative contribution of different Ca2+ signals mediated by CP-AMPARs and mGluRs to LTP induction also remains to be determined. To answer these questions we examined the hypotheses that in O/A interneurons, distinct dendritic Ca2+ signals produced by CP-AMPARs and mGluRs (1) are localized to particular dendritic microdomains, (2) demonstrate specific properties, and (3) are differentially regulated by synaptic activity.
Using two-photon calcium imaging and whole-cell recordings in O/A interneurons, we identified three distinct types of NMDA- and VGCC-independent postsynaptic currents and Ca2+ transients that are localized within distinct dendritic microdomains and dependent on the glutamate receptor class involved (ionotropic versus metabotropic). Using different paradigms of synaptic stimulation, we found that both CP-AMPAR- and mGluR-mediated Ca2+ signals were evoked within given dendritic microdomains, but they were differentially regulated by presynaptic and postsynaptic activity. Furthermore, the properties of mGluR-mediated Ca2+ signals were consistent with the Hebbian requirements for LTP induction at interneuron synapses.
Materials and Methods
Slice preparation. Transverse hippocampal slices were obtained from 15- to 23-d-old Sprague Dawley rats (Charles River, St. Laurent, Quebec, Canada). Animals were deeply anesthetized with halothane. After decapitation, the brain was rapidly removed into ice-cold, oxygenated “cutting” solution containing (in mm): 250 sucrose, 2 KCl, 1.25 NaH2PO4, 26 NaHCO3, 7 MgSO4, 0.5 CaCl2, and 10 glucose, pH 7.4, 320-340 mOsm. Slices (300 μm thick) were cut with a Vibratome (Leica VT1000S; Leitz, Wetzlar, Germany), transferred to a heated (35°C) oxygenated solution containing (in mm): 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 3 MgSO4, 1 CaCl2, and 10 glucose, and allowed to cool down to room temperature for 30 min. Slices were allowed to recover for at least 1 h before experiments.
Whole-cell recording. During experiments, slices were perfused continuously (2.5 ml/min) with artificial CSF (ACSF) containing (in mm): 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 MgSO4, 2 CaCl2, and 10 glucose at 25°C. In some experiments (see Figs. 7, 8), ACSF contained lower Mg2+ (1 mm) and higher Ca2+ (3 mm) concentrations. CA1 interneurons of stratum oriens/alveus were identified with the aid of an infrared camera (70 Series; Dage-MTI, Michigan City, IN) mounted on an upright microscope (Axioskop 2FS; Carl Zeiss, Kirkland, Quebec, Canada) equipped with a long-range water-immersion objective. Whole-cell voltage-clamp recordings were made from somata using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). Recording pipettes (4-5 MΩ) were filled with a solution containing (in mm): 130 CsMeSO3, 5 CsCl, 2 MgCl2, 5 diNa-phosphocreatine, 10 HEPES, 2 ATP2Na, 0.4 GTPNa, 0.1 spermine, 2 N-ethyl bromide quaternary salt, and 0.2 Oregon Green-488-BAPTA-1-hexapotassium salt (OGB-1) (KD = 170 nm) (Molecular Probes, Eugene, OR) or 0.2 Fluo-5F (KD = 2.3 μm) (Molecular Probes), pH 7.25-7.35, 275-285 mOsm. Cells were voltage clamped at -60 mV unless stated otherwise. Membrane currents were low-pass filtered at 2 kHz, digitized at 10 kHz, and stored on a microcomputer using a data acquisition board (Digidata1322A; Axon Instruments) and pClamp8 software (Axon Instruments).
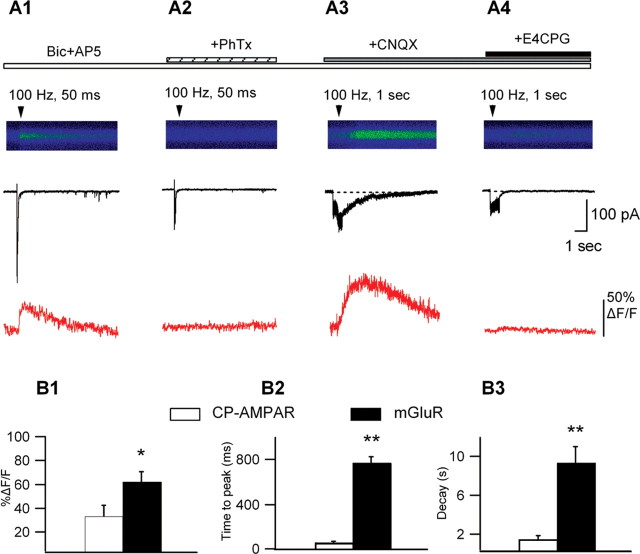
Figure 7.
Differential regulation by presynaptic activity of synaptically elicited Ca2+ signals mediated by CP-AMPARs and mGluRs. A, Line scan images of synaptically evoked Ca2+ signals with representative currents (black) and associated Ca2+ transients (red) from the same cell in the presence of Bic and AP5 (A1), after PhTx block (A2), in the presence of Bic, AP5, and CNQX (A3), and after adding E4CPG (A4). Single bursts of high-frequency stimulation (30 μA; 5 stimuli at 100 Hz) produced summated EPSCs with appreciable Ca2+ influx (A1). These Ca2+ transients were completely blocked by PhTx (A2). In the absence of ionotropic glutamate transmission (in Bic, AP-5, and CNQX), repetitive synaptic stimulation (150 μA; 0.5-1 s at 100 Hz) evoked slower postsynaptic inward currents accompanied by slow Ca2+ transients (A3) that were both antagonized by E4CPG (A4). B, Bar graphs of the peak amplitude (B1), time-to-peak (B2), and decay time (B3) of synaptically evoked Ca2+ transients mediated by CP-AMPARs (PhTx sensitive; i.e., from A1) and by mGLuRs (E4CPG sensitive; i.e., from A3) (n = 5; *p < 0.05; **p < 0.01).
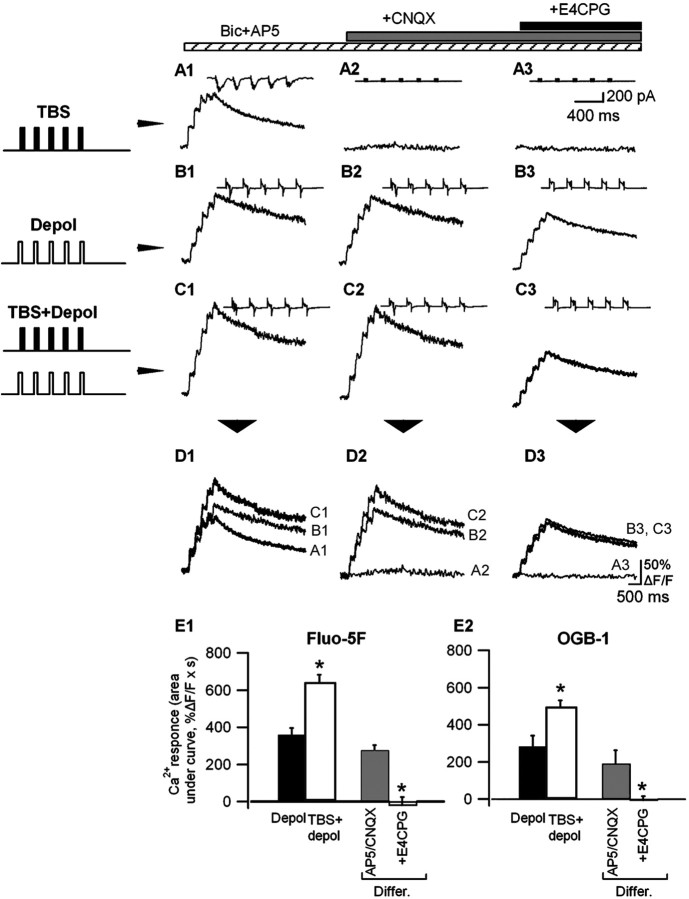
Figure 8.
Regulation of Ca2+ signals mediated by mGluRs by postsynaptic depolarization. A1-C3, Representative Ca2+ transients measured with Fluo-5F and associated currents (insets) evoked by TBS at -60 mV (A1, A2), postsynaptic depolarization (-20 mV) (B1, B2), and TBS paired with depolarization (C1, C2) in different pharmacological conditions. D, Superimposed Ca2+ transients elicited in the same cell by the three stimulation protocols, showing that Ca2+ transients associated with TBS plus depolarization (C1, C2) were larger than those elicited by TBS (A1, A2) or depolarization (B1, B2) alone and that this difference was blocked by the mGluR antagonist E4CPG (B3, C3). E, Bar graphs of mean Ca2+ responses measured with Fluo-5F (E1) (n = 4 cells) and OGB-1 (E2) (n = 4 cells) evoked by depolarization alone (Depol), pairing TBS and depolarization (TBS + depol), and the difference in response between the two conditions (Differ.), showing that this difference was completely blocked by E4CPG (*p < 0.05).
Ca2 imaging of interneuron dendrites. After obtaining the whole-cell configuration, 20-30 min were allowed for intracellular diffusion of the fluorophore. Imaging was performed using a multiphoton confocal laser scanning microscope LSM 510 (Carl Zeiss) with a mode-locked Ti:Sapphire laser operated at 780 nm wavelength, 76 MHz pulse repeat, <200 fs pulse width and pumped by a solid-state source (Mira 900 and 5 W Verdi argon ion laser; Coherent, Santa Clara, CA). A long-range water-immersion objective (40×; numerical aperture, 0.8) was used. Fluorescence was detected through a long-pass filter (cutoff, 505 nm), and images were acquired using the LSM 510 software (Carl Zeiss). Fluorescence signals were collected by scanning a line along the dendrite of interest (total length, ∼5-20 μm), resulting in a high temporal (3 ms per line) resolution (see Fig. 1). Fluorescence transients were measured at 30-100 μm from the soma. Recordings were not made from axon-bearing segments of dendrites. The image focus of the line was checked carefully and adjusted occasionally for possible drift. Electrophysiological recordings and Ca2+ imaging were typically stable for ∼1 h.
Figure 1.

Micropressure application of glutamate to interneuron dendrites produces local Ca2+ signals via multiple mechanisms. A, Multiphoton image (z-stack) of 200 optical sections taken at 0.5 μm intervals of an Oregon Green-filled interneuron; dendritic region of interest (ROI) near a puff-pipette is indicated by the box. B, Reconstruction of the same biocytin-filled interneuron. C, Pseudocolor images of an enlarged ROI with basal dendritic fluorescence (C1) and after glutamate puff (C2). C3, Dashed line indicates position for the line scan shown in D. D, Line scan images of the responses to glutamate puff (Glut. puff; C2). (arrowhead) with dashed lines bordering the region of interest for fluorescence measurements. Traces below are glutamate-evoked dendritic Ca2+ transients (red) and postsynaptic currents recorded from soma (black) (average of 4 sweeps). D2, Residual responses are the Ca2+ transient and membrane current independent of NMDARs and VGCCs [elicited in the presence of TTX (0.5 μm), Bic (10 μm), AP-5 (50 μm), and Ni2+ (50 μm)]. E, Spatiotemporal pattern of glutamate-evoked Ca2+ signals from a different cell; a dendritic ROI (E1), through which a line scan was placed, is indicated by the brackets. The amplitude of the calcium signal (ΔF/F) was measured from the corresponding line scan image (E2, top) and plotted as a function of distance along the dendrite for different times after glutamate application: 100 ms (red), 600 ms (green), 1100 ms (blue), and 1600 ms (black) (E2, bottom). These plots were well fitted by Gaussians. The x-axis of the plot (E2, bottom) corresponds to the length of the line scan image (E2, top). Note that in E2 (top) only the initial 1000 ms of the line scan is illustrated.
Pharmacological and synaptic stimulation. Direct activation of dendritic postsynaptic receptors was achieved by local micropressure pulses of glutamate (1 mm; 5-10 psi; 3-30 ms) via a glass pipette (tip diameter of 2-3 μm) connected to a pressure application system (PicoSpritzer II; Parker Instrumentation, Fairfield, NJ) and positioned ∼10 μm above the OGB-1-filled dendrite. Local synaptic stimulation of interneurons was performed using an extracellular pipette (2-3 MΩ) filled with ACSF and positioned in the immediate vicinity (<15 μm) of the dendrite of interest. To achieve the precise positioning of puff- and stimulation pipettes, tips of pipettes were marked with the concentrated water-insoluble dye DiL (Molecular Probes). Active dendritic spots were identified by imaging the frames containing the target dendritic region of interest before and during glutamate puff or electrical stimulation (see Fig. 1).
Chemicals. Mechanisms of glutamate-evoked postsynaptic currents and associated Ca2+ transients were explored in the presence of tetrodotoxin (TTX) (0.5 μm), bicuculline (Bic) (10 μm), dl-2-amino-5-phosphonovaleric acid (AP-5) (50 μm) or (+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate (MK-801) (15 μm), NiCl2 (50 μm), and CdCl2 (100 μm) (Sigma, St. Louis, MO). All compounds were prepared in advance as stock solutions, frozen at -20°C, diluted on the day of experiment, and bath applied. In some experiments the AMPAR/kainate receptor (KAR) antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (20 μm), the Ca2+-permeable AMPAR antagonist philanthotoxin-433 Tris-trifluoroacetate (PhTx) (10 μm) (RBI, Natick, MA), the group I/II mGluR antagonist (RS)-α-ethyl-4-carboxyphenyl-glycin (E4CPG) (500 μm) (Tocris, Ellisville, MO), or the mGluR1a antagonist (S)(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385) (100 μm) (Tocris) was added to the extracellular solution.
Data analysis. Calcium measurements and electrophysiological recordings were analyzed using the LSM 510, Clampfit 9.0 software (Axon Instruments), and IgorPro (Wavemetrics, Lake Oswego, OR). For analysis of calcium transients, the fluorescence background was subtracted from the fluorescence intensity averaged over the line. Changes in fluorescence were calculated relative to the baseline (from 1 s before stimulation) and expressed as %ΔF/F = [(F - Frest)/Frest] × 100. For comparison of voltage dependence of postsynaptic currents and Ca2+ transients from different cells, data derived from a single neuron held at a different membrane potential (-80 to +60 mV) were normalized to values at -60 mV. The rectification index (RI) of the inwardly rectifying I-V relationship was defined as the ratio of the recorded current amplitude at +40 mV to the predicted linear value at +40 mV (extrapolated from linear fit of the currents at the negative potentials) (Liu and Cull-Candy, 2000; Lei and McBain, 2002, 2004). The time-to-peak of the calcium signal was calculated as the time from the stimulation to the peak amplitude. Decay kinetics of membrane currents and calcium transients were fitted using single or double exponential fitting algorithms of IgorPro. Summary data are expressed as mean ± SE. Statistical significance between groups was determined using a two-tailed Student's t test or ANOVA followed by appropriate post hoc tests (Student-Newman-Keuls, Dunnet, or Tukey-Kramer).
Histology. Biocytin (0.15-0.2%) (Sigma) was routinely added to the internal patch solution to allow cell labeling and reconstruction. Visualization of biocytin was performed as described previously (Perez et al., 2001). Three-dimensional light microscopic reconstructions were performed using an Eclipse E600W microscope (Nikon, Tokyo, Japan) equipped with a digital camera (Retiga 1300; QImaging, Burnaby, British Columbia, Canada). Final stacks of cell images (see Fig. 1 B) were merged in two-dimension in Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA).
Results
Glutamate-evoked local dendritic Ca2+ transients in O/A interneurons
To investigate the relative contribution of ionotropic and metabotropic glutamate receptors to dendritic Ca2+ rises in O/A interneurons, we first examined postsynaptic currents and Ca2+ transients evoked by glutamate micropressure application to interneuron dendrites (Fig. 1). Interneurons were recorded in whole-cell voltage-clamp mode and filled with the fluorescent Ca2+ indicator OGB-1 (Fig. 1A). Biocytin cell labeling confirmed that all cells recorded were CA1 interneurons with their soma located in stratum oriens near the alveus and horizontally oriented sparsely spiny dendrites (n = 52). In 17 of these cells, the axonal arborization was stained and projected to stratum lacunosum-moleculare (Fig. 1B). Such cells are known to be somatostatin-positive interneurons (Freund and Buzsaki, 1996) that express high levels of mGluR1a extrasynaptically and perisynaptically (Masu et al., 1991; Baude et al., 1993). After an initial dye-loading period of 20-30 min, interneuron dendrites were identified with confocal imaging, and a puff-pipette was positioned ∼10 μm above the dendrite of interest (Fig. 1 A). Micropressure pulses of glutamate (1 mm) evoked a local increase in fluorescence in the dendrite of interest (Fig. 1C1,C2). To measure Ca2+ transients in the region of interest, a line was positioned along the dendrite (Fig. 1C3), and Ca2+ signals were imaged in the line-scanning mode while glutamate-evoked membrane currents were recorded in the soma (Fig. 1D).
Ca2+ signals evoked by such discrete applications of glutamate were highly localized to individual dendritic compartments (Fig. 1C2). To quantitatively examine the spatial spread of Ca2+ signals, we plotted the amplitude of the Ca2+ signal (%ΔF/F) as a function of distance along the dendrite, at distinct times after glutamate application (Fig. 1E). These traces were well fitted by Gaussian curves (Fig. 1E2), and the spatial extent of a Ca2+ signal at a given time was determined from the SD of the Gaussian curve (Goldberg et al., 2003c). At the peak amplitude of Ca2+ transients, which on average occurred at ∼100 ms after glutamate application, Ca2+ signals were highly localized (σpeak = 2.53 ± 0.36 μm). The maximal resolvable spatial extent of the Ca2+ signal (σmax), which occurred at ∼1600 ms after glutamate application, was 3.94 ± 1.32 μm(n = 10). The spatiotemporal spread of Ca2+ signals in our experiments was larger than those associated with spontaneous CP-AMPAR-mediated EPSPs (<1 μm) (Goldberg et al., 2003c), suggesting the activation of both synaptic and extrasynaptic receptors by glutamate application.
Ca2+ transients, induced in ACSF at -60 mV (Fig. 1D1), consisted of NMDAR- and VGCC-mediated components, because they were significantly reduced in the presence of 50 μm AP-5 and 50 μm Ni2+ (Fig. 1D2) (control, 108 ± 11% ΔF/F; AP-5 + Ni2+, 50.3 ± 6.9% ΔF/F; n = 25; p < 0.001; paired t test). A significant component of Ca2+ signal was dependent on NMDARs (43 ± 14% of total Ca2+ response; range, 25-75%; n = 10), whereas a smaller contribution was attributable to Ni2+-sensitive VGCCs (10 ± 2% of total Ca2+ response; range, 6-15%; n = 5). Importantly, we observed a significant component of Ca2+ signals independent of NMDAR/VGCC (47 ± 7% of total Ca2+ response; n = 25) (Fig. 1D2). To examine the mechanisms of these NMDAR- and VGCC-independent Ca2+ signals, all subsequent experiments were conducted in the presence of 50 μm AP-5 (or MK-801), 50 μm Ni2+, and 100 μm Cd2+. The combination of Ni2+ and Cd2+ completely blocked Ca2+ transients elicited by membrane depolarization (-20 mV, 50 ms; n = 3 cells; data not shown).
Dendritic Ca2+ signals arising from CP-AMPAR activation
Activation of two types of glutamate receptors, apart from NMDARs, has been linked to Ca2+ transients in interneurons: CP-AMPARs (Isa et al., 1996) and group I mGluRs (Woodhall et al., 1999; Gee and Lacaille, 2004). It is well established that endogenous polyamines block CP-AMPARs and endow them with the property of inward rectification (Bowie and Mayer, 1995; Donevan and Rogawski, 1995; Kamboj et al., 1995; Koh et al., 1995; Washburn and Dingledine, 1996). To discriminate between responses mediated by CP-AMPAR and mGluRs, we examined the I-V relationship of glutamate-evoked membrane currents (in the presence of TTX, Bic, AP-5, Ni2+, and Cd2+). We observed that membrane currents and their associated Ca2+ responses could be separated in three types based on the rectification index. The first type of response elicited by local glutamate application consisted of inwardly rectifying currents (RI < 0.3) with a reversal potential of 0 mV (Fig. 2A) (n = 5). Dendritic Ca2+ transients associated with this type of current declined steeply in amplitude with membrane depolarization (Fig. 2B). Comparison of peak Ca2+ transients at -60 and -20 mV showed that Ca2+ signals were significantly diminished at depolarized membrane potential (to 52 ± 5.1% ΔF/Fnorm; p < 0.05; n = 5).
Figure 2.

CP-AMPARs mediate dendritic Ca2+ transients associated with inwardly rectifying glutamate currents. A, Representative membrane currents (average of 4 sweeps) independent of NMDARs and VGCCs (in TTX, Bic, AP-5, Ni2+, and Cd2+) evoked by glutamate at different membrane potentials (Vm, -80 to +60 mV; left) and average I-V relationship for all cells (n = 5) with inward rectification (right). Before averaging, data from individual cells were normalized to the current amplitude at -60 mV. B, Associated Ca2+ transients (average of 4 sweeps) at different Vm (left) and plots of average peak Ca2+ transients for all cells as a function of Vm, showing steeply declining voltage dependence of Ca2+ transients (right). Data at different voltages were also normalized to Ca2+ transients at -60 mV. C, An example of use-dependent block of inwardly rectifying currents (left graph) by PhTx in one cell. Representative traces of membrane currents and associated Ca2+ transients before (Ctl) and after PhTx block are shown at right. D, Bar graph of the average amplitude of current and Ca2+ transient for all cells in the same conditions, showing that CP-AMPARs mediated this type of current and Ca2+ transients (n = 5; *p < 0.05).
To verify that CP-AMPARs mediate those responses, we used the high-affinity and use-dependent antagonist of CP-AMPARs, PhTx (10 μm) (Toth and McBain, 1998). After an initial period of recording of control responses, the slice was incubated in a solution containing PhTx (10 μm) for 10 min. After this period, stimulation by glutamate was resumed, and current amplitude as well as peak Ca2+ transient were blocked in a use-dependent manner during the next 20 min (Fig. 2C,D). Thus, dendritic Ca2+ signals associated with inwardly rectifying glutamate currents are mediated by CP-AMPARs in O/A interneurons.
Dendritic Ca2+ signals mediated by mGluRs and CP-AMPARs
The second type of glutamate-evoked membrane currents consisted of responses with intermediate inward rectification (0.3 < RI < 0.7) (Fig. 3A). The mean reversal potential of these currents was 10.1 ± 3.8 mV (n = 6). These responses appeared to be composed of fast and slow components, but because both components overlapped to a large extent, it was difficult to separate them (Fig. 3A, left). Dendritic Ca2+ transients associated with these currents showed a slightly declining voltage relationship (Fig. 3B), such that they did not show significant changes with membrane depolarization from -60 to -20 mV (decrease to 84.6 ± 15.6% ΔF/Fnorm; p > 0.05; n = 6). To test the idea that this type of response resulted from activation of mixed non-NMDARs and mGluRs, we examined their sensitivity to the AMPAR/KAR antagonist CNQX (20 μm) and the group I/II mGluR antagonist E4CPG (500 μm) (Fig. 3C). Bath application of CNQX significantly reduced the amplitude of membrane currents and Ca2+ transients, indicating that AMPARs/KARs partly mediated these responses. The residual membrane currents and Ca2+ transients observed in CNQX were fully blocked by addition of E4CPG (p < 0.05; n = 5) (Fig. 3C), showing that group I/II mGluRs also partly mediated these responses. Thus, a combination of AMPARs/KARs and group I/II mGluRs were involved in the Ca2+ transients associated with membrane currents showing intermediate inward rectification. The more pronounced block of these responses by CNQX indicates a larger contribution of AMPARs/KARs over mGluRs in the intermediate type of membrane currents and their associated Ca2+ signals.
Figure 3.
Dendritic Ca2+ signals produced by joint activation of AMPARs/KARs and group I/II mGluRs. A, Representative glutamate-evoked membrane currents (average of 4 sweeps) at different Vm (left) and average I-V relationship for all cells (n = 6) showing intermediate inward rectification (right). B, Associated Ca2+ transients (average of 4 sweeps) at different Vm (left) and plots of average peak Ca2+ transients as a function of Vm, indicating slightly declining voltage dependence of Ca2+ transients (right). C, Representative currents and Ca2+ transients (top) evoked by glutamate puffs in control (Ctl) and in the presence of the AMPA/KAR antagonist (CNQX) and the group I/II mGluR antagonist (E4CPG), and bar graphs (bottom) of the average amplitude of current (left) and Ca2+ transient (right) in the same conditions. Currents and Ca2+ transients were sensitive to CNQX and E4CPG, indicating that a combination of AMPA/KARs and group I/II mGluRs participate in these responses (n = 6; *p < 0.05; **p < 0.01).
Local dendritic Ca2+ signals via group I mGluRs
The third type of glutamate-evoked membrane currents consisted of linear and outwardly rectifying membrane currents (RI >0.7). The mean reversal potential of these currents was 11.3 ± 3.5 mV (Fig. 4A)(n = 14). Occasionally, this type of current also appeared to consist of fast and slow components, but they were usually overlapping and undistinguishable. Ca2+ transients associated with this type of current demonstrated a bell-shaped relation with voltage reaching maximal values near -20 mV (Fig. 4B). Comparison of peak Ca2+ transients at -60 and -20 mV showed that Ca2+ transients significantly increase with membrane depolarization (to 156.2 ± 22.5% ΔF/Fnorm; p < 0.05; n = 14). The Ca2+ transients associated with these membrane currents were recorded in the presence of Ni2+ and Cd2+ and thus did not likely implicate VGCCs. To test whether this type of current and Ca2+ transients were mediated by AMPARs/KARs and group I/II mGluRs, we examined their pharmacological sensitivity to CNQX and E4CPG (Fig. 4C). Bath application of CNQX inhibited membrane currents, indicating that they were mediated partly by AMPARs/KARs (p < 0.05; n = 5) (Fig. 4C); however, addition of CNQX did not significantly affect Ca2+ transients (p = 0.275; n = 5) (Fig. 4C). The residual current uncovered in the presence of CNQX demonstrated outward rectification and significantly slower rise time (control, 25.8 ± 2.4 ms; CNQX, 138.8 ± 28.8 ms; p < 0.05; n = 5) and decay (control, 189.5 ± 33.1 ms; CNQX, 749.0 ± 165.3 ms; p < 0.05; n = 5). The Ca2+ transient associated with this residual current significantly increased with membrane depolarization (to 143 ± 17.9% ΔF/Fnorm; p < 0.05; n = 5). The residual currents and Ca2+ transients observed in CNQX were fully blocked by E4CPG, showing that these responses were mediated by the group I/II mGluRs (p < 0.05; n = 5) (Fig. 4C). Because O/A interneurons express high levels of the mGluR1a subtype of group I mGluRs (Masu et al., 1991; Baude et al., 1993), we also examined the effect of the selective mGluR1a antagonist LY367385. In the presence of LY367385, Ca2+ transients were reduced significantly (control, 37.5 ± 10.3% ΔF/F; LY367385, 12.5 ± 5.8% ΔF/F; p < 0.05, n = 4), whereas membrane currents were not affected (control, -35.3 ± 8.5 pA; LY367385, -39.8 ± 19.9 pA; p = 0.749; n = 4). These results indicate that AMPARs/KARs and group I/II mGluRs are differentially involved in outwardly rectifying currents and associated Ca2+ transients. Ca2+-impermeable AMPARs/KARs and group I/II mGluRs contribute to membrane currents, whereas only group I/II mGluRs (including mGluR1a) participate in their associated Ca2+ transients. Moreover, these results uncover a characteristic voltage relationship of mGluR-mediated membrane currents and Ca2+ transients resulting in potentiation of these responses by membrane depolarization in the absence of Ca2+ influx through VGCCs.
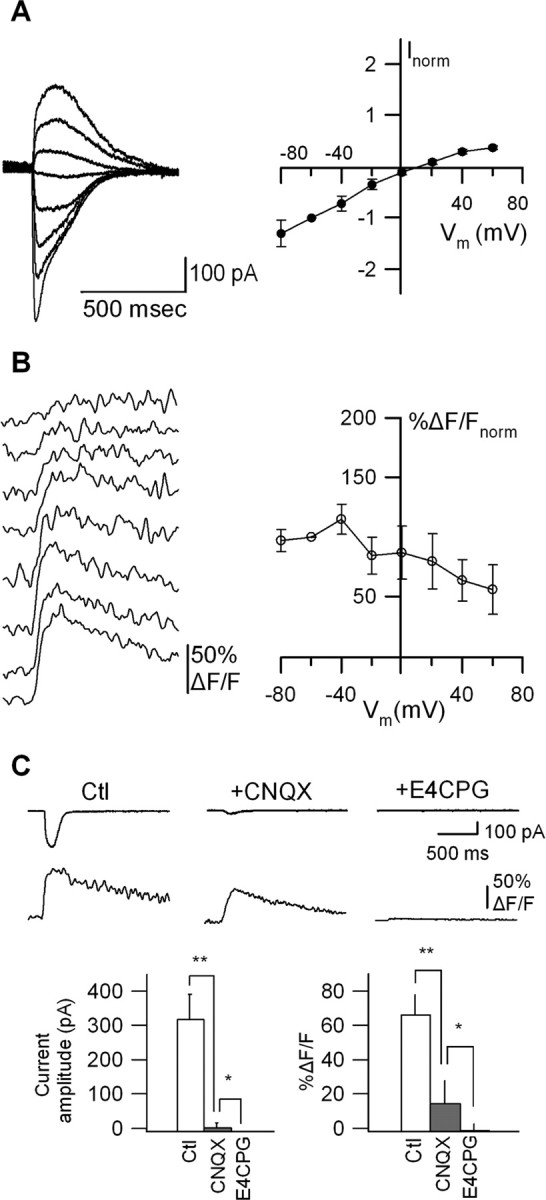
Figure 4.
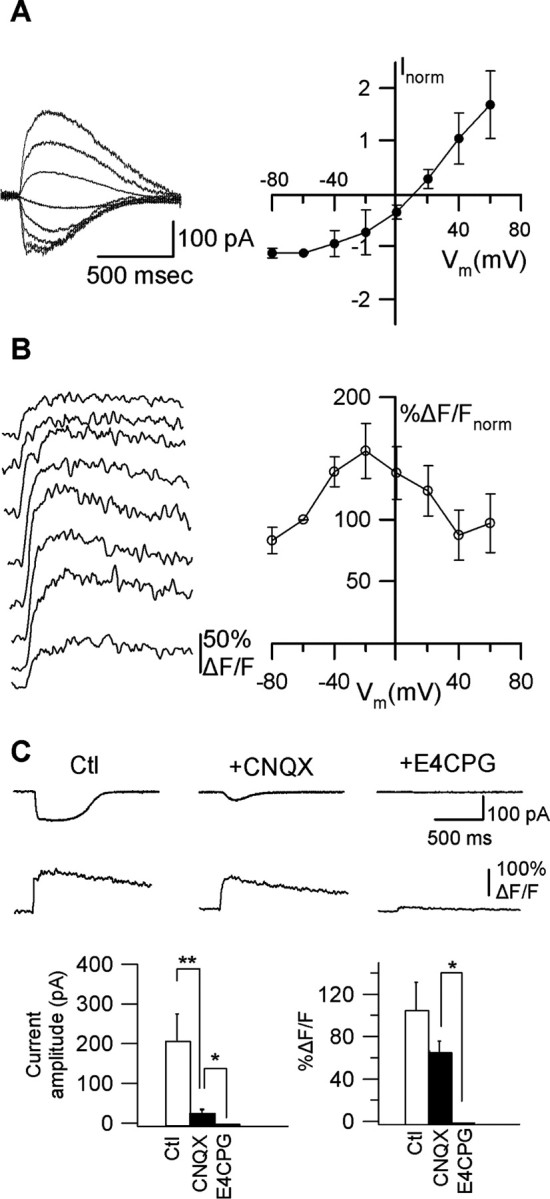
Dendritic Ca2+ signals mediated by group I/II mGluRs. A, Representative postsynaptic currents (average of 4 sweeps) at different Vm (left) and average I-V relationship for all cells (n = 14) with outward rectification (right). B, Associated Ca2+ transients (average of 4 sweeps) at different Vm (left) and plots of average peak Ca2+ transients for all cells as a function of Vm, showing bell-shaped voltage dependence of Ca2+ transients (right). C, Representative currents and Ca2+ transients evoked by glutamate puffs (top) in control (Ctl) and in the presence of the AMPA/KAR antagonist (CNQX) and the group I/II mGluR antagonist (E4CPG), and bar graphs (bottom) of the average amplitude of current (left) and Ca2+ transient (right) in the same conditions. Currents were sensitive to CNQX and E4CPG, whereas Ca2+ transients were significantly reduced only by E4CPG, showing that AMPA/KARs and group I/II mGluRs contribute to membrane currents, whereas only mGluRs mediate Ca2+ transients (n = 5; *p < 0.05; **p < 0.01).
The time course of dendritic Ca2+ signal depends on the glutamate receptor type involved
Having found that glutamate-evoked dendritic Ca2+ signals in O/A interneurons occur through multiple mechanisms, we next examined the time course of membrane currents and Ca2+ signals associated with each response type (Fig. 5). Inwardly rectifying membrane currents and associated Ca2+ signals (type I) demonstrated the fastest kinetics (current rise time, 19.1 ± 1.8 ms; current decay time, 85.2 ± 16.9 ms; Ca2+ transient time-to-peak, 62.6 ± 16.3 ms; Ca2+ transient decay time, 503.2 ± 75.5 ms; n = 5). In contrast, outwardly rectifying membrane currents and their Ca2+ transients (type III) had a significantly slower time course (current rise time, 44.5 ± 9.7 ms; current decay time, 197.5 ± 23.1 ms; Ca2+ transient time-to-peak, 191.6 ± 14.1 ms; Ca2+ transient decay, 1763.8 ± 386.9 ms; n = 14) than responses with inward rectification (type I) (p < 0.05; ANOVA). Responses with intermediate inward rectification (type II) also exhibited a fast rise time (current rise time, 14.1 ± 2.6 ms; Ca2+ transient time-to-peak, 66.3 ± 19.8 ms; n = 6), but their decay was intermediate (current decay time, 117.9 ± 34.9 ms; Ca2+ transient decay, 1323.8 ± 391.5 ms; n = 6) and not significantly different from that of type I and III responses. Because OGB-1, used in these experiments, is a relatively high-affinity Ca2+ indicator and could affect the kinetics of Ca2+ transients, we examined Ca2+ signals using a lower affinity Ca2+ indicator, Fluo-5F (Fig. 5C). Pharmacologically isolated (in Bic + AP-5 + E4CPG) CP-AMPAR-mediated Ca2+ transients were significantly faster (time-to-peak, 52.6 ± 11.7 ms; decay, 443.3 ± 123.4 ms; n = 5) than those mediated by mGluRs (in Bic + AP-5 + CNQX) (time-to-peak, 220.6 ± 25.5 ms; decay, 1281.6 ± 93.4 ms; n = 5). Thus, membrane currents and Ca2+ signals with faster kinetics were associated with CP-AMPARs, whereas those with slower kinetics were linked with mGluRs.
Figure 5.
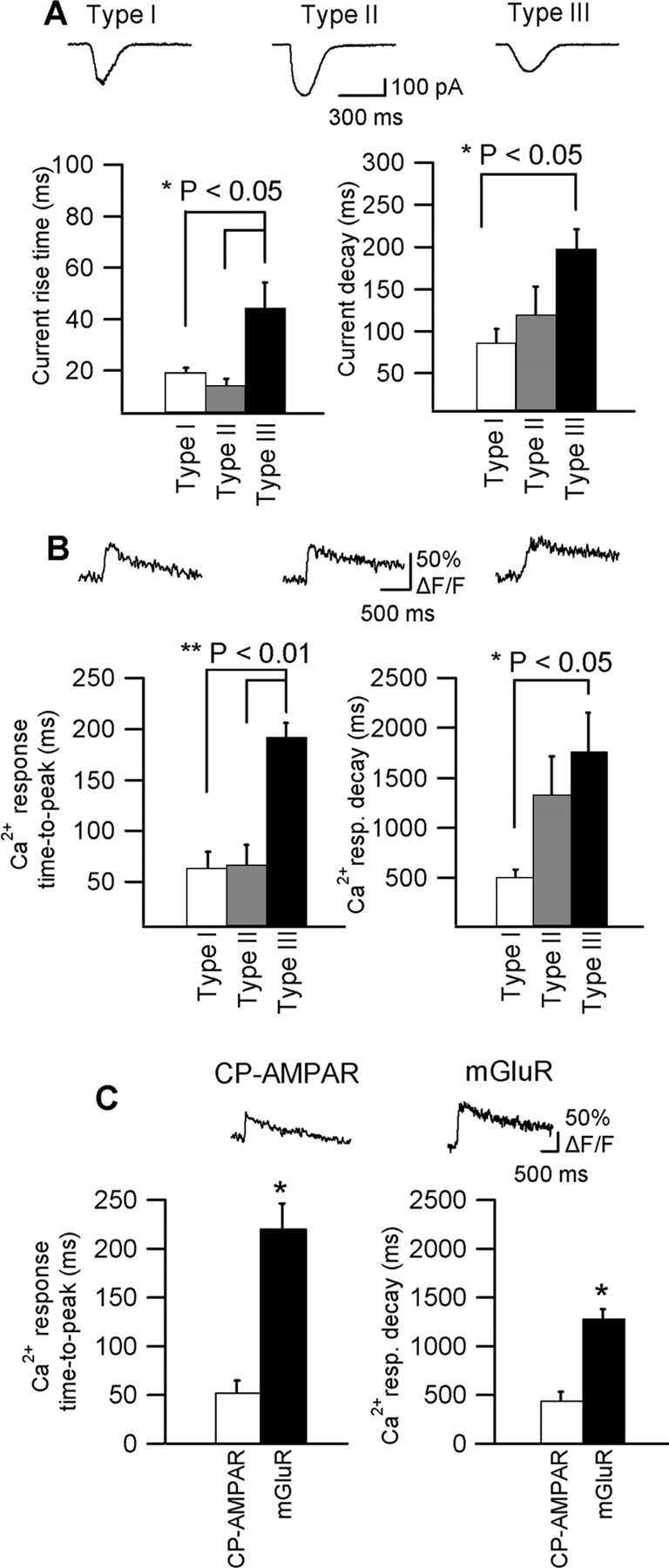
Response-specific time course. A, Representative examples of three types of glutamate-evoked currents (Vh =-60 mV): type I (inwardly rectifying), type II (intermediate), and type III (outwardly rectifying), and bar graphs of the mean rise (left) and decay times (right) of currents for the three response types for all cells. B, Ca2+ transients associated with the three types of membrane currents shown in A and bar graphs of the mean time-to-peak (left) and decay time (right) of Ca2+ transients for all cells. Membrane currents and Ca2+ transients of type I and II responses displayed significantly faster rise time than those of type III response. Type III response showed significantly slower decay than type I response (**p < 0.01; *p < 0.05; ANOVA). C, Representative examples of CP-AMPAR-mediated (in AP-5 + E4CPG) and mGluR-mediated (in AP-5 + CNQX) Ca2+ transients measured with the low-affinity Ca2+ indicator Fluo-5F; bar graphs indicate the mean time-to-peak (left) and decay time (right) of Ca2+ transients for all cells (CP-AMPAR, n = 5; mGluR, n = 5).
Distinct Ca2+ signals at different dendritic sites of single interneurons
To examine in more detail the spatial segregation of different Ca2+ signals, we measured Ca2+ transients at different dendritic sites of the same interneuron. First, distinct Ca2+ signals could arise at different dendritic sites separated by short distances (<5 μm) in a given cell (n = 3 cells). As shown in Figure 6A, applying glutamate to a given dendritic region elicited different responses at two adjacent dendritic sites (Fig. 6A, sites 1 and 2). The Ca2+ signal measured within the first microdomain was unaffected by NMDAR, VGCC, and AMPAR/KAR antagonists and likely mediated by mGluRs (Fig. 6A, red trace). In contrast, the Ca2+ transient measured at the second site was decreased by AP-5 and Ni2+ and blocked by subsequent addition of CNQX (Fig. 6A, blue trace), suggesting that NMDAR, VGCC, and AMPAR/KAR activation contributed to this response. Thus, distinct Ca2+ microdomains involving ionotropic and metabotropic glutamate receptors can be closely localized but spatially segregated in interneuron dendrites.
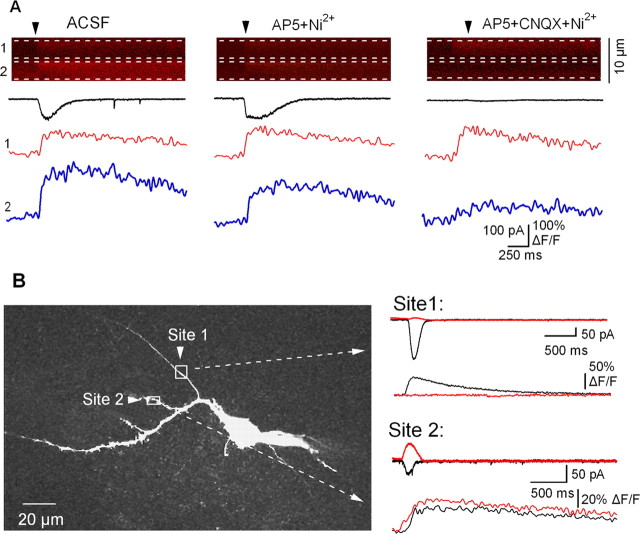
Figure 6.
Distinct Ca2+ signals in different dendritic sites of single interneurons. A, Representative line scan images of responses to glutamate applications (arrowhead). Dashed lines indicate the regions of interest for fluorescence measurements. Traces below illustrate glutamate-evoked currents at -60 mV (black) and associated dendritic Ca2+ transients measured within microdomains 1 (red) and 2 (blue). Distinct Ca2+ signals were produced in the two adjacent microdomains. At site 1 (red trace) the Ca2+ response was independent of NMDARs, VGCCs, and AMPA/KARs and was likely mediated by mGluRs, whereas at site 2 (blue trace) the Ca2+ response consisted of components mediated by NMDARs/VGCCs and AMPARs/KARs. B, Z-stack of an Oregon Green-filled interneuron (left) showing the two different locations of the puff-pipette and two dendritic regions of interest (1 and 2) indicated by the boxes. The corresponding membrane currents and Ca2+ transients evoked in each dendritic region at two Vm [Vh = -60 mV (black) and +40 mV (red)] are illustrated at right. The membrane currents at site 1 showed inward rectification, whereas those at site 2 showed an outward I-V relationship. The currents and Ca2+ transients are an average of four sweeps.
To examine whether distinct Ca2+ signals mediated by different glutamate receptors can arise at distant dendritic sites in a given interneuron, we evoked membrane currents and Ca2+ transients with glutamate applications to two different dendritic branches of the same cell (n = 5 cells) (Fig. 6B). Ca2+ signals were measured in branches of the same diameter and at a similar distance from the soma (40-60 μm). Membrane currents evoked by glutamate at different dendritic sites of the same cell displayed different rectification: (1) inward and intermediate rectification (type I/type II; n = 2 cells), (2) inward and linear/outward rectification (type I/type III; n = 2 cells) (Fig. 6B), or (3) only inward rectification at both sites (type I/type I; n = 1 cell). The distinct membrane currents were also associated with different Ca2+ signals. As illustrated in the representative cell in Figure 6, membrane currents evoked by glutamate at the first dendritic site showed inward rectification (RI = 0.06) (Fig. 6B). This response was associated with large Ca2+ signal at -60 mV (61% ΔF/F) and no detectable transient at +40 mV. These response properties are consistent with the mediation of membrane currents and Ca2+ signals by CP-AMPARs. Currents evoked by glutamate at the second dendritic site showed an outward I-V relation (RI = 1.6) (Fig. 6B). They were associated with Ca2+ signals of smaller amplitude at -60 mV (31% ΔF/F) than at +40 mV (44% ΔF/F). These response properties are consistent with a contribution of Ca2+-impermeable (CI)-AMPAR/KARs and mGluRs to these membrane currents and Ca2+ signals. The elicitation of differently rectifying membrane currents and heterogeneous Ca2+ transients by glutamate application at different dendritic sites of single interneurons suggests a differential distribution of specific combination of ionotropic and metabotropic glutamate receptors at specific dendritic sites. This differential distribution of glutamate receptors suggests a dendritic site-specific Ca2+ signaling function in O/A interneurons.
Synaptically activated Ca2+ signals mediated by CP-AMPARs and mGluRs
To investigate the mechanisms of synaptically activated dendritic Ca2+ signals, we examined Ca2+ transients associated with EPSCs evoked by low-intensity stimulation (∼30 μA; 50 μs; five stimuli at 100 Hz; Vh =-60 mV). Such stimulation elicited local Ca2+ responses associated with compound EPSCs of large amplitude, suggesting that Ca2+ transients and synaptic currents were generated at overlapping dendritic sites.
Comparison of Ca2+ transients in the presence and absence of AP-5 indicated that synaptic Ca2+ transients included an NMDAR-dependent component (43.3 ± 11.5% ΔF/F; n = 6). NMDAR-independent Ca2+ signals (in AP-5) (Fig. 7A1) were observed in 8 of 14 cells, and they were of small amplitude (33.1 ± 8.1% ΔF/F), had relatively fast kinetics (time-to-peak, 70.4 ± 11.8 ms; decay time, 1418 ± 437 ms), and were fully blocked by PhTx (n = 8) (Fig. 7A2). PhTx significantly reduced EPSCs but did not block them completely, suggesting that CP-AMPARs and CI-AMPARs were involved in the generation of EPSCs; however, because synaptic activation of CI-AMPARs did not produce any detectable Ca2+ signals, low-intensity synaptic stimulation activated dendritic Ca2+ signals solely mediated by NMDARs and CP-AMPARs.
We next investigated in the same cells whether an increase in intensity and duration of synaptic stimulation could recruit group I/II mGluR-mediated EPSCs. In the absence of ionotropic glutamatergic transmission (in CNQX and AP-5), a gradual increase in stimulus strength (up to 150-200 μA) evoked EPSCs and Ca2+ transients that were sensitive to E4CPG in two of eight cells (data not shown). Thus, release of glutamate by a short burst of stimulation can occasionally be sufficient to activate mGluR-mediated postsynaptic currents and Ca2+ transients. Because increased presynaptic activity was required to activate group I mGluRs and elicit postsynaptic responses in CA1 pyramidal cells (Congar et al., 1997), we examined the effect of longer trains of stimuli. Tetanic stimulation (150 μA; 0.3-1 s at 100 Hz; Vh = -60 mV) evoked slower EPSCs and Ca2+ transients (time-to-peak, 770.2 ± 51.7 ms; decay time, 9337 ± 1637 ms) (Fig. 7A3) that were blocked by E4CPG (n = 8) (Fig. 7A4). The amplitude of the synaptically activated mGluR-mediated Ca2+ signals was significantly larger than that mediated by CP-AMPARs (mGluRs, 62.2 ± 8.4% ΔF/F; n = 8; p < 0.05). Thus, synaptically activated Ca2+ signals that are independent of NMDARs were mediated in part by CP-AMPARs and group I/II mGluRs. Moreover, these different components were present in the same dendritic microdomains but were elicited by different stimulation paradigms. These results indicate that dendritic Ca2+ signaling in O/A interneurons is differentially regulated by a presynaptic activity pattern: short duration presynaptic activity involves only CP-AMPARs, whereas longer-duration repetitive synaptic activity will lead to the additional recruitment of mGluRs. Moreover, the increase in presynaptic activity will change Ca2+ transients from small, fast signals to large, slow responses within given dendritic microdomains.
mGluR-mediated Ca2+ signals induced by pairing synaptic stimulation with postsynaptic depolarization
Because a stimulation protocol pairing repetitive stimulation with postsynaptic depolarization induces a mGluR1a- and Ca2+-dependent LTP in O/A interneurons (Perez et al., 2001; Lapointe et al., 2004), we examined whether CP-AMPAR- and mGluR-mediated Ca2+ signals were differentially activated by pairing synaptic theta-burst stimulation (TBS) [five bursts at 200 ms intervals (5 Hz), each burst consisting of four pulses at 100 Hz] with depolarization (five 60-ms-long depolarizing steps to -20 mV). We measured Ca2+ signals activated by the pairing protocol in two recording conditions, using Ca2+ indicators with low-affinity Fluo-5F (Fig. 8A-D,E1) and high-affinity OGB-1 (Fig. 8E2). As expected from using a low-affinity indicator (Helmchen, 1999), we observed larger peak calcium signals that were not saturated during the pairing protocol with Fluo-5F (Fig. 8A-D). In the presence of Bic and AP-5 and at a holding potential of -60 mV, TBS alone produced Ca2+ transients that were fully blocked by CNQX and thus likely mediated by CP-AMPARs (Fig. 8A1,A2). Postsynaptic depolarization alone produced Ca2+ spikes and Ca2+ transients that were not sensitive to CNQX but slightly reduced by the group I/II mGluR antagonist E4CPG (Fig. 8B). The pairing of TBS with depolarization evoked Ca2+ transients that summated sublinearly (Fig. 8D) and were not reduced in CNQX (Fig. 8C1,C2), demonstrating that CP-AMPARs do not provide significant Ca2+ influx during the paired stimulation protocol; however, E4CPG significantly decreased Ca2+ signals induced by TBS paired with membrane depolarization (Fig. 8C3). Moreover, in AP-5 and CNQX, Ca2+ transients evoked by TBS paired with depolarization were significantly larger using both Ca2+ indicators [Fluo-5F area under curve, 634.9 ± 45.1% ΔF/F · s (Fig. 8D2); OGB-1, 486.9 ± 43.8% ΔF/F · s] than the Ca2+ rises produced by postsynaptic depolarization alone [Fluo-5F area under curve, 358.6 ± 37.1% ΔF/F · s (Fig. 8D2); OGB-1, 281.3 ± 58.2% ΔF/F · s; n = 8; p < 0.05; ANOVA]. Furthermore, with both indicators, the difference between Ca2+ signals induced by TBS paired with postsynaptic depolarization and those produced by the depolarization alone (in AP-5 and CNQX) was fully blocked by E4CPG (n = 8; p < 0.05; paired t test) (Fig. 8E). Because TBS alone did not produce any Ca2+ signals in CNQX, an additional component of Ca2+ transients was produced by TBS paired with postsynaptic depolarization and mediated by mGluRs. Thus, Ca2+ transients appearing to summate sublinearly during the pairing protocol (Fig. 8D) were not a result of summation of Ca2+ transients evoked by TBS alone and depolarization alone but were caused by another additional mGluR-mediated component recruited by the pairing paradigm. These results indicate that TBS paired with postsynaptic depolarization is an effective protocol to elicit dendritic Ca2+ signals mediated by mGluRs in O/A interneurons and that group I/II mGluR-mediated Ca2+ mechanisms may effectively signal coincident presynaptic and postsynaptic activity.
Discussion
Our experiments led to four main findings regarding dendritic Ca2+ signaling via glutamate receptors in hippocampal O/A interneurons. First, these cells express CP-AMPARs providing fast Ca2+ signals. Second, individual cells can coexpress CP-AMPARs, CI-AMPARs, and/or group I/II mGluRs (including mGluR1a), producing heterogeneous Ca2+ signals in different dendritic sites. Third, activation of CI-AMPARs did not elicit detectable Ca2+ signals, but these receptors were often associated with mGluRs, resulting in longer-lasting Ca2+ signals. Fourth, CP-AMPAR- and mGluR-mediated Ca2+ signals demonstrate distinct voltage dependence and are differentially regulated by presynaptic and postsynaptic activity within the same dendritic microdomain. These findings indicate that differential activation of specific glutamate receptor-mediated Ca2+ signals within spatially restricted dendritic microdomains serve distinct signaling functions and allow for multiple forms of Ca2+-mediated synaptic plasticity in these cells. The specific properties of mGluR-mediated Ca2+ signals are consistent with the Hebbian requirements for LTP induction in O/A interneurons.
Heterogeneity of Ca2+ signals in dendrites of O/A interneurons
Our findings and other recent studies provide new insights into the complexity of Ca2+ signaling in interneuron dendrites (Gee et al., 2001; Kaiser et al., 2001, 2004; Goldberg et al., 2003a,b,c). In neocortical interneurons, synaptic Ca2+ signals were highly localized within dendritic microdomains and mediated by NMDARs or CP-AMPARs (Goldberg et al., 2003a,b,c; Kaiser et al., 2004). We provide novel evidence that in hippocampal O/A interneurons, multiple Ca2+ signals mediated by NMDARs, CP-AMPARs, and mGluRs coexist within highly restricted dendritic compartments. NMDAR-mediated Ca2+ transients were variable in amplitude in O/A interneurons, consistent with their variable dendritic NMDAR distribution (Nyiri et al., 2003). In pyramidal cells, NMDAR-mediated Ca2+ signals underlie long-term potentiation (Malenka et al., 1988; Malenka and Nicoll, 1999), but this may not be the case in O/A interneurons (Maccaferri and McBain, 1996). Interestingly, in hippocampal lucidum interneurons, activation of NMDARs at synapses colocalizing CI-AMPARs leads to long-term depression (LTD) (Lei and McBain, 2002, 2004). For O/A interneurons, however, a role of NMDARs in such LTD remains to be determined.
Our data uncovered significant contributions of CP-AMPARs to dendritic calcium signaling in O/A interneurons. CP-AMPAR-mediated Ca2+ transients exhibited fast kinetics, allowing for calcium compartmentalization in aspiny interneuron dendrites, as in neocortical fast-spiking cells (Goldberg et al., 2003c); however, this property of CP-AMPARs may be prominent mostly near resting membrane potentials, because CP-AMPAR-mediated Ca2+ signaling diminished with depolarization. In contrast, mGluR-mediated Ca2+ transients were enhanced by membrane depolarization and demonstrated slower kinetics. Thus, the different properties of CP-AMPAR- and mGluR-mediated Ca2+ signals suggest different signaling roles for these Ca2+ mechanisms in O/A interneurons. Together, these results highlight the heterogeneity of Ca2+ signals in O/A interneuron dendrites and suggest multiple distinct Ca2+ signaling functions at interneuron synapses.
Heterogeneous targeting of CP-AMPARs, CI-AMPARs, and mGluRs
Our findings indicate major differences in dendritic Ca2+ signals elicited by glutamate and synaptic stimulation. With glutamate application, we found three types of Ca2+ signals: (1) via CP-AMPARs, (2) via mixed activation of CP-AMPARs and mGluRs, and (3) via mGluRs. In contrast, synaptic Ca2+ responses were mediated by both CP-AMPARs and mGluRs within given dendritic microdomains. Because glutamate stimulation may involve synaptic and extrasynaptic receptors whereas synaptic stimulation may implicate only synaptic receptors, our observations suggest that CP-AMPARs and group I/II mGluRs (1) are colocalized at synaptic sites and (2) can be found independently at extrasynaptic sites. Heterogeneous glutamate-evoked currents and Ca2+ signals were observed in different dendritic microdomains of a given cell, indicating a heterogeneous distribution of AMPARs and mGluRs within individual cells. Interestingly, CI-AMPARs were more often associated with mGluRs than CP-AMPARs. Thus, longer-lasting Ca2+ signals may occur in dendritic microdomains colocalizing CI-AMPARs and mGluRs. Such heterogeneity in Ca2+ mechanisms suggests distinct, site-specific Ca2+ signaling roles in dendritic microdomains with different combinations of CP-AMPARs, CI-AMPARs, and mGluRs.
Our Ca2+ imaging and electrophysiological results also show that CP-AMPARs and CI-AMPARs can be (1) colocalized at some dendritic microdomains or (2) targeted independently at others, indicating a differential targeting of AMPARs with variable GluR2 composition to specific postsynaptic sites within individual cells. This is consistent with preliminary evidence that AMPAR-mediated synaptic currents have different types of I-V relationships in O/A interneurons (Croce and Lacaille, 2004), as reported in CA3 stratum lucidum interneurons (Toth and McBain, 1998; Lei and McBain, 2002). Surprisingly, we found that at synapses composed of both CP-AMPARs and CI-AMPARs, Ca2+ signals were mediated solely by CP-AMPARs. This observation is at odds with the partial Ca2+ permeability of CI-AMPARs reported previously (PCa/PNa > 0) (Geiger et al., 1995). Our results suggest that the Ca2+ permeability of synaptically activated CI-AMPARs in situ is negligible or below our detection threshold.
Differential regulation of CP-AMPAR- and mGluR-mediated Ca2+ transients
We found that each synaptic component of Ca2+ transients could be uncovered with different stimulation. Weak stimulation produced dendritic Ca2+ signals via CP-AMPARs, whereas higher intensity or repetitive stimulation recruited group I/II mGluRs. Thus, synaptic CP-AMPAR- and mGluR-mediated Ca2+ signals are differentially regulated by presynaptic activity. The recruitment of group I/II mGluRs by intense and repetitive synaptic stimulation is consistent with their perisynaptic localization (Baude et al., 1993; Lujan et al., 1996). In cerebellar stellate cells, high presynaptic activity produced downregulation of CP-AMPARs (Liu and Cull-Candy, 2000, 2002). Such AMPAR regulation by presynaptic activity has not been reported in O/A interneurons, but because they receive intense synaptic activity (McBain, 1995; Maccaferri and Lacaille, 2003), it represents another possible regulatory mechanism at these synapses.
Our finding that synaptic stimulation paired with depolarization results in group I/II mGluR-dependent Ca2+ transients provides clear evidence of a regulation of mGluR-mediated Ca2+ signal by postsynaptic activity. Enhancement of mGluR-mediated currents and Ca2+ signals by membrane depolarization was reported for pyramidal cells (Luthi et al., 1997; Chuang et al., 2000; Rae et al., 2000; Rae and Irving, 2004). Recently, mGluR1a-mediated EPSCs in O/A interneurons were reported to depend on depolarization causing Ca2+ entry via VGCCs (Huang et al., 2004); however, in our experiments, the bell-shaped voltage relationship of glutamate-evoked Ca2+ transients mediated by mGluRs was observed in Ni2+ and Cd2 mediated currents may result from activation of nonselective cation channels (Congar et al., 1997; Gee et al., 2003). These currents are modulated by divalent cations and demonstrate outward rectification (Congar et al., 1997). Interestingly, in heterologous systems, currents mediated by heteromultimeric channels consisting of transient-receptor-potential (TRP) subunits, TRPC1 and TRPC5, demonstrate properties similar to those of mGluR-mediated currents in our conditions (Strübing et al., 2001). Given that a TRPC1-mGluR1a interaction underlies mGluR-mediated EPSCs in Purkinje cells (Kim et al., 2003), a similar TRPC-mGluR1a relation may be present in O/A interneurons. Preliminary evidence suggests that Ca2+ signals in O/A interneurons involve multiple mechanisms differentially coupled to mGluR1 and mGluR5 as well as to Ca2+ influx via TRPCs and intracellular Ca2+ release (Topolnik et al., 2004). Interestingly, our observation that depolarization-induced Ca2+ signals were partly inhibited by mGluR antagonists suggests that, in addition, VGCCs may be modulated positively by basal mGluR activation.
Implication for long-term synaptic plasticity in interneurons
CP-AMPARs are prevalent in interneurons (McBain and Dingledine, 1993; Jonas et al., 1994; Geiger et al., 1995; Koh et al., 1995; Isa et al., 1996). Ca2+ influx via these receptors is involved in multiple forms of interneuron synaptic plasticity (Mahanty and Sah, 1998; Laezza et al., 1999; Liu and Cull-Candy, 2000, 2002; Toth et al., 2000; Ross and Soltesz, 2001; Lei and McBain, 2002). Short-term facilitation of CP-AMPAR-mediated currents results from the use- and voltage-dependent relief of the polyamine block (Rozov et al., 1998; Toth et al., 2000). CP-AMPAR-dependent LTD in hippocampal interneurons is a form of plasticity expressed presynaptically but induced postsynaptically. Moreover, it is induced at near resting membrane potentials but not at depolarized potentials (Laezza et al., 1999; Lei and McBain, 2002, 2004). The negative voltage relationship of CP-AMPAR-mediated Ca2+ signals found in the present study is consistent with these LTD induction requirements because these responses were reduced in amplitude by membrane depolarization. These LTD induction mechanisms are in contrast to those of mGluR-dependent LTP induction in interneurons, which are Hebbian in nature and require presynaptic activation and postsynaptic depolarization (Perez et al., 2001). The bell-shaped voltage relationship of mGluR-dependent Ca2+ responses revealed here is compatible with these LTP induction requisites because these signals were uncovered by depolarization paired with stimulation. Thus, the LTD induction protocol is optimal for eliciting CP-AMPAR-mediated Ca2+ signals, whereas the LTP induction protocol is best for evoking mGluR-dependent Ca2+ transients. Ca2+-dependent LTD- versus LTP-induction mechanisms in interneurons therefore may be determined by the specific CP-AMPAR- versus mGluR-mediated Ca2+ mechanisms associated with each stimulation paradigm. Thus, the direction of synaptic plasticity appears to be a function of the specific Ca2+ signaling mechanism activated in interneuron dendritic microdomains. Our findings uncover heterogeneous computational capabilities of local dendritic Ca2+ signaling with fast CP-AMPAR-mediated Ca2+ transients playing a role as coincidence detectors of synaptic inputs (Goldberg et al., 2003c) and slow mGluR-mediated Ca2+ responses signaling coincident presynaptic and postsynaptic activity in O/A interneurons.
Footnotes
This work was supported by the Human Frontier Science Program, Canadian Institutes of Health Research, and Fonds de la Recherche en Santé du Québec. J.-C.L. is the recipient of a Canada Research Chair in Cellular and Molecular Neurophysiology. We thank Stephanie Ratté for comments on this manuscript, Yury Kovalchuk for advice on imaging techniques, Dominique Nouel for technical assistance, France Morin for help with biocytin labeling, and Dimitry Topolnik for cell reconstructions.
Correspondence should be addressed to Dr. Jean-Claude Lacaille, Département de Physiologie, Centre de Recherche en Sciences Neurologiques, Université de Montréal, Case Postale 6128, Succursale Centre-Ville, Montréal, Quebec, Canada H3C 3J7. E-mail: jean-claude.lacaille@umontreal.ca.
P. Congar's present address: Institute National de la Recherche Agronomique, Domaine de Vilvert, Unité de Neurobiologie de l'Olfaction et de la Prise Alimentaire, Bātiment Biotechnologies, 78352 Jouy-en-Josas, Cedex, France.
Copyright © 2005 Society for Neuroscience 0270-6474/05/250990-12$15.00/0
References
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P (1993) The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron 11: 771-787. [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML (1995) Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15: 453-462. [DOI] [PubMed] [Google Scholar]
- Carmant L, Woodhall G, Ouardouz M, Robitaille R, Lacaille JC (1997) Interneuron-specific Ca2+ responses linked to metabotropic and ionotropic glutamate receptors in rat hippocampal slices. Eur J Neurosci 9: 1625-1635. [DOI] [PubMed] [Google Scholar]
- Chuang SC, Bianchi R, Wong RK (2000) Group I mGluR activation turns on a voltage-gated inward current in hippocampal pyramidal cells. J Neurophysiol 83: 2844-2853. [DOI] [PubMed] [Google Scholar]
- Congar P, Ben-Ari Y, Crepel V (1997) A long-lasting calcium-activated nonselective cationic current is generated by synaptic stimulation or exogenous activation of group I metabotropic glutamate receptors in CA1 pyramidal neurons. J Neurosci 17: 5366-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce A, Lacaille J-C (2004) Different mechanisms of transmission at two functional types of excitatory synapses composed of AMPA and kainate receptors in hippocampal oriens-alveus interneurons. Soc Neurosci Abstr 30: 404.6. [Google Scholar]
- Donevan SD, Rogawski M (1995) Intracellular polyamines mediate inward rectification of Ca-permeable AMPA receptors. Proc Natl Acad Sci USA 92: 9298-9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G (1996) Interneurons of the hippocampus. Hippocampus 6: 347-470. [DOI] [PubMed] [Google Scholar]
- Gee CE, Lacaille JC (2004) Group I metabotropic glutamate receptor actions in oriens-alveus interneurons of rat hippocampal CA1 region. Brain Res 1000: 92-101. [DOI] [PubMed] [Google Scholar]
- Gee CE, Woodhall G, Lacaille JC (2001) Synaptically activated calcium responses in dendrites of hippocampal oriens-alveus interneurons. J Neurophysiol 85: 1603-1613. [DOI] [PubMed] [Google Scholar]
- Gee CE, Benquet P, Gerber U (2003) Group I metabotropic glutamate receptors activate a calcium-sensitive transient receptor potential-like conductance in rat hippocampus. J Physiol (Lond) 546: 655-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H (1995) Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15: 193-204. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Yuste R, Tamas G (2003a) Ca2+ imaging of mouse neocortical interneurone dendrites: Ia type K+ channels control action potential backpropagation. J Physiol (Lond) 551: 49-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Yuste R, Tamas G (2003b) Ca2+ imaging of mouse neocortical interneurone dendrites: contribution of Ca2+-permeable AMPA and NMDA receptors to subthreshold Ca2+ dynamics. J Physiol (Lond) 551: 67-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Tamas G, Aronov D, Yuste R (2003c) Calcium microdomains in aspiny dendrites. Neuron 40: 807-821. [DOI] [PubMed] [Google Scholar]
- Helmchen F (1999) Dendrites as biochemical compartments. In: Dendrites (Stuart G, Spruston N, Hausser M, eds), pp 161-192. Oxford: Oxford UP.
- Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE (2004) Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J Neurosci 24: 4551-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Itazawa S, Iino M, Tsuzuki K, Ozawa S (1996) Distribution of neurones expressing inwardly rectifying and Ca(2+)-permeable AMPA receptors in rat hippocampal slices. J Physiol (Lond) 491: 719-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Burnashev N (1995) Molecular mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron 15: 987-990. [DOI] [PubMed] [Google Scholar]
- Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H (1994) Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron 12: 1281-1289. [DOI] [PubMed] [Google Scholar]
- Kaiser KM, Zilberter Y, Sakmann B (2001) Back-propagating action potentials mediate calcium signaling in dendrites of bitufted interneurons in layer 2/3 of rat somatosensory cortex. J Physiol (Lond) 535: 17-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser KM, Lubke J, Zilberter Y, Sakmann B (2004) Postsynaptic calcium influx at single synaptic contacts between pyramidal neurons and bitufted interneurons in layer II/III of rat neocortex is enhanced by backpropagating action potentials. J Neurosci 24: 1319-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG (1995) Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol (Lond) 486: 297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia R, Worley P, Linden D (2003) Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature 426: 285-291. [DOI] [PubMed] [Google Scholar]
- Koh DS, Burnashev N, Jonas P (1995) Block of native Ca(2+)-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol (Lond) 486: 305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Doherty JJ, Dingledine R (1999) Long-term depression in hippocampal interneurons: joint requirement for pre- and postsynaptic events. Science 285: 1411-1414. [DOI] [PubMed] [Google Scholar]
- Lapointe V, Morin F, Ratte S, Croce A, Conquet F, Lacaille JC (2004) Synapse-specific mGluR1-dependent long-term potentiation in interneurons regulates mouse hippocampal inhibition. J Physiol (Lond) 555: 125-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, McBain CJ (2002) Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron 33: 921-933. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ (2004) Two loci of expression for long-term depression at hippocampal mossy-fiber-interneuron synapses. J Neurosci 24: 2112-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SG, Cull-Candy SG (2000) Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature 405: 454-458. [DOI] [PubMed] [Google Scholar]
- Liu SG, Cull-Candy SG (2002) Activity-dependent change in AMPA receptor properties in cerebellar stellate cells. J Neurosci 22: 3881-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P (1996) Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci 8: 1488-1500. [DOI] [PubMed] [Google Scholar]
- Luthi A, Gahwiler BH, Gerber U (1997) 1S,3R-ACPD induces a region of negative slope conductance in the steady-state current-voltage relationship of hippocampal pyramidal cells. J Neurophysiol 77: 221-228. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Lacaille JC (2003) Interneuron diversity series: hippocampal interneuron classifications—making things as simple as possible, not simpler. Trends Neurosci 26: 564-571. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ (1996) Long-term potentiation in distinct subtypes of hippocampal nonpyramidal neurons. J Neurosci 16: 5334-5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty NK, Sah P (1998) Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature 394: 683-687. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA (1999) Long-term potentiation—a decade of progress? Science 285: 1870-1874. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Zucker RC, Nicoll RA (1988) Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science 242: 81-84. [DOI] [PubMed] [Google Scholar]
- Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S (1991) Sequence and expression of a metabotropic glutamate receptor. Nature 349: 760-765. [DOI] [PubMed] [Google Scholar]
- McBain CJ (1995) Hippocampal inhibitory neuron activity in the elevated potassium model of epilepsy. J Neurophysiol 73: 2853-2863. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Dingledine R (1993) Heterogeneity of synaptic glutamate receptors on CA3 stratum radiatum interneurones of rat hippocampus. J Physiol (Lond) 462: 373-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Stephenson FA, Freund TF, Somogyi P (2003) Large variability in synaptic N-methyl-d-aspartate receptor density on interneurons and a comparison with pyramidal-cell spines in the rat hippocampus. Neuroscience 119: 347-363. [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Lacaille JC (2001) A Hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci USA 98: 9401-9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae MG, Irving AJ (2004) Both mGluR1 and mGluR5 mediate Ca2+ release and inward currents in hippocampal CA1 pyramidal neurons. Neuropharmacology 46: 1057-1069. [DOI] [PubMed] [Google Scholar]
- Rae MG, Martin DJ, Collingridge GL, Irving AJ (2000) Role of Ca2+ stores in metabotropic l-glutamate receptor-mediated supralinear Ca2+ signaling in rat hippocampal neurons. J Neurosci 20: 8628-8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ST, Soltesz I (2001) Long-term plasticity in interneurons of the dentate gyrus. Proc Natl Acad Sci USA 98: 8874-8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Burnashev N (1999) Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature 401: 594-598. [DOI] [PubMed] [Google Scholar]
- Rozov A, Zilberter Y, Wollmuth LP, Burnashev N (1998) Facilitation of currents through rat Ca2+-permeable AMPA receptor channels by activity-dependent relief from polyamine block. J Physiol (Lond) 511: 361-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE (2001) TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29: 645-655. [DOI] [PubMed] [Google Scholar]
- Topolnik L, Azzi M, Lacaille J-C (2004) Involvement of Src/MAPK cascade in fast mGluR1a- but not slow mGluR5-mediated Ca2+ signals in hippocampal interneurons. Soc Neurosci Abstr 30: 162.12. [Google Scholar]
- Toth K, McBain CJ (1998) Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci 1: 572-578. [DOI] [PubMed] [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ (2000) Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci 20: 8279-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Dingledine R (1996) Block of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J Pharmacol Exp Ther 278: 669-678. [PubMed] [Google Scholar]
- Woodhall G, Gee CE, Robitaille R, Lacaille JC (1999) Membrane potential and intracellular Ca2+ oscillations activated by mGluRs in hippocampal stratum oriens/alveus interneurons. J Neurophysiol 81: 371-382. [DOI] [PubMed] [Google Scholar]