Figure 7.
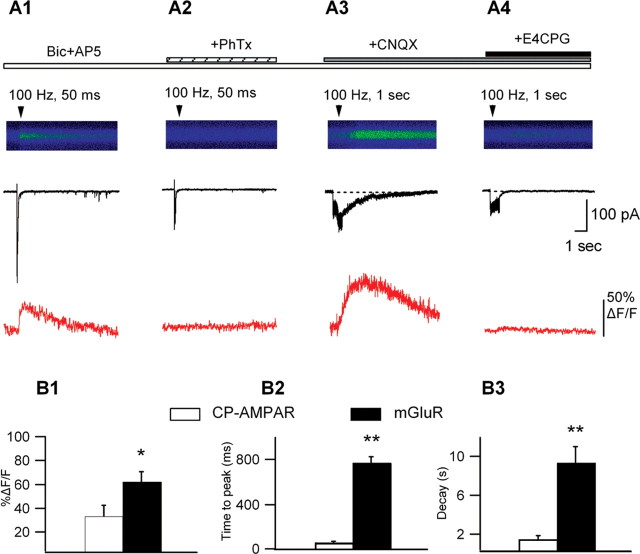
Differential regulation by presynaptic activity of synaptically elicited Ca2+ signals mediated by CP-AMPARs and mGluRs. A, Line scan images of synaptically evoked Ca2+ signals with representative currents (black) and associated Ca2+ transients (red) from the same cell in the presence of Bic and AP5 (A1), after PhTx block (A2), in the presence of Bic, AP5, and CNQX (A3), and after adding E4CPG (A4). Single bursts of high-frequency stimulation (30 μA; 5 stimuli at 100 Hz) produced summated EPSCs with appreciable Ca2+ influx (A1). These Ca2+ transients were completely blocked by PhTx (A2). In the absence of ionotropic glutamate transmission (in Bic, AP-5, and CNQX), repetitive synaptic stimulation (150 μA; 0.5-1 s at 100 Hz) evoked slower postsynaptic inward currents accompanied by slow Ca2+ transients (A3) that were both antagonized by E4CPG (A4). B, Bar graphs of the peak amplitude (B1), time-to-peak (B2), and decay time (B3) of synaptically evoked Ca2+ transients mediated by CP-AMPARs (PhTx sensitive; i.e., from A1) and by mGLuRs (E4CPG sensitive; i.e., from A3) (n = 5; *p < 0.05; **p < 0.01).