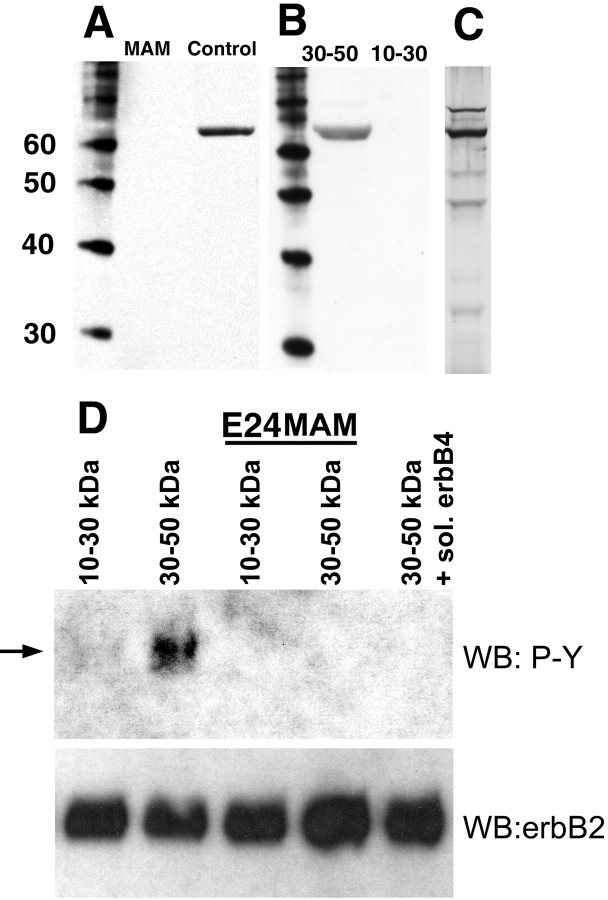
Figure 1.
The fraction of medium conditioned by ferret cortical slices, which has radializing activity, contains NRG1. A, Conditioned media obtained from normal organotypic cultures of ferret cortex were fractioned into aliquots of different molecular weight by centrifugation and then subjected to Western blot analysis with NRG1 antibodies. NRG1 immunoreactivity was observed in the 30–50 kDa fraction from the conditioned media obtained from normal (control) but not E24 MAM (MAM)-treated organotypic cultures. MW markers are indicated in the left lane. B, In the conditioned media obtained from normal cortical slices, the 30–50 kDa fraction was immunoreactive for NRG1, whereas the 10–30 kDa fraction was not. MW markers are shown in the left lane. C, A silver stain on a gel obtained from the normal 30–50 kDa conditioned media fraction. The molecular weights correspond to the markers shown in A and B. D, L6 muscle cells in culture were treated with conditioned media fractions 30–50 and 10–30 kDa at eight slice equivalents/milliliter for 5 min (see Materials and Methods for details). Cells were lysed and subjected to Western blot analysis with anti-phosphotyrosine antibodies (WB:P-Y). Only the fraction that possesses the radial glia-orienting activity (30–50 kDa fraction obtained from normal tissue) induced the tyrosine phosphorylation of an 185 kDa protein (arrow), similar to that induced by NRG1. Neither the inactive fraction from normal tissue nor either molecular weight fraction from MAM-treated tissue induced phosphorylation. Addition of a soluble form of the erbB4 receptor also blocked activity from the normal active fraction. The blots were stripped and subjected to immunoblotting with erbB2 to demonstrate equivalent loading (WB: erbB2). sol., Soluble.