Figure 5.
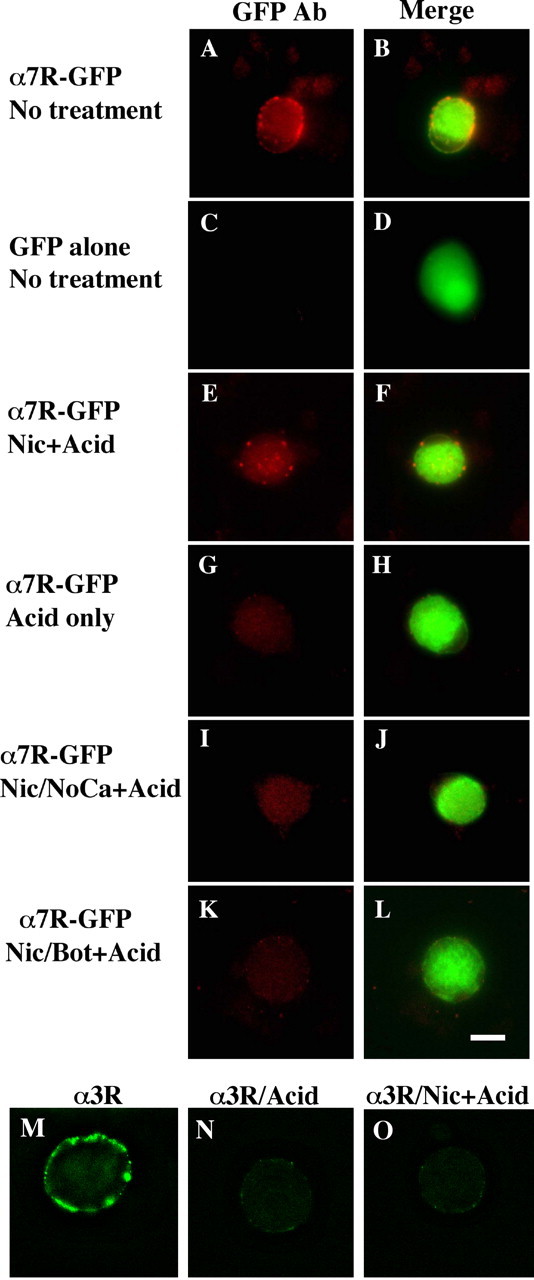
Nicotine-induced internalization of surface α7-nAChRs in a SNARE-dependent manner. E8 CG neurons were grown in culture for 6-7 d after being transfected with constructs encoding either the fusion protein α7-nAChR-GFP (α7R-GFP) (A, B, E-L) or GFP alone (C, D). The cells were then treated as indicated below and either imaged directly (A-D) or acid-stripped and then processed for imaging (E-L). The left panel of each pair shows immunostaining with anti-GFP antibody in red, and the right panel shows the image merged with the green GFP fluorescence to yield yellow. Treatments are as in Figure 4: A-D, no treatment; E, F, nicotine plus acid stripping (+Acid); G, H, negative control, no nicotine plus acid; I, J, nicotine plus acid without extracellular calcium; K, L, nicotine plus acid after Bot C1. The α7-nAChR-GFP fusion protein appears in the surface membrane where it can be stained with anti-GFP antibody; nicotine drives internalization of the receptor (revealed by protection of bound anti-GFP antibody against acid stripping) in a calcium-dependent manner that is blocked by botulinum C1. In other cultures, α3*-nAChRs on the cell surface were labeled with mAb 35, and after rinsing, the cells were imaged after fixation, permeabilization, and labeling with fluorescent secondary antibody (M); or acid-washed first to remove mAb 35 and then fixed, permeabilized, and labeled (N); or nicotine treated to induce internalization, acid-washed, and then fixed, permeabilized, and labeled (O). In contrast to α7-nAChRs, the nicotine treatment did not protect mAb 35 labeling against acid-wash, indicating that no detectable α3*-nAChR/antibody complex had been internalized. Similar results were obtained in two additional experiments. Scale bar, 10 μm.
