Figure 1.
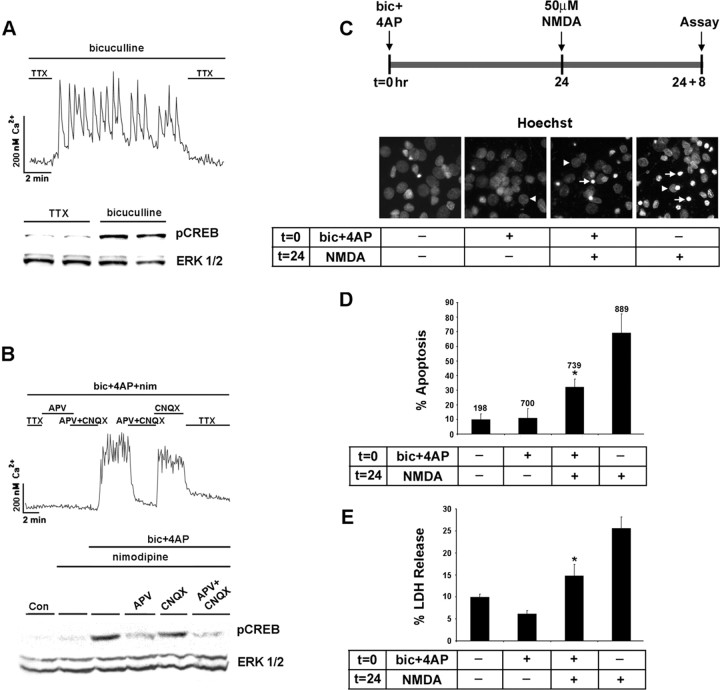
The NMDA receptor couples synaptic activity to the Ser133 phosphorylated form of CREB. A, Top, Initially, cortical neurons cultured for 10 d were placed in a HEPES-based buffer containing TTX (1 μm), and internal Ca2+ levels were monitored by using fura-2 time-lapse digital microscopy. In the presence of bicuculline (50 μm), the withdrawal of the TTX from the buffer elicited rapid and reproducible Ca2+ transients. A, Bottom, Relative to cortical cultures maintained in TTX, 10 min of synaptic activity elicited by the removal of TTX trigged a marked increase in pCREB expression. As a protein loading control, this blot was stripped and probed for total ERK expression. Duplicate determinations are shown for each condition. B, Top, Driving the synaptic network via activation of the NMDA receptor. To examine the necessity of L-type Ca2+ channels and ionotropic glutamate receptors in synaptic activity-dependent CREB phosphorylation, we placed cortical cultures in HEPES buffer containing bicuculline (50 μm), 4-AP (200 μm), and nimodipine (5 μm). In the majority of neurons, the basal Ca2+ levels were maintained by the withdrawal of TTX (1 μm) and the addition of APV (100 μm) to the perfusion medium. Washout of APV and the non-NMDA ionotropic receptor antagonist CNQX (10 μm) triggered a robust and sustained increase in Ca2+ levels. Ca2+ levels also were increased by the withdrawal of APV, indicating that the synaptic release of glutamate stimulated Ca2+ influx via an NMDA receptor-mediated mechanism. B, Bottom, Western blot analysis for pCREB revealed that the NMDA receptor coupled synaptic activity to pCREB expression. C, Synaptic activity attenuates NMDA-induced cell death. Hoechst labeling was used to monitor the effects that synaptic activity has on NMDA-evoked neurotoxicity. A bout (15 min) of synaptic activity was elicited by the application of bicuculline and 4-AP (bic+4AP) in the presence of nimodipine and CNQX at time 0 (t = 0). Then 24 h later (t = 24), the cultures were exposed to NMDA (50 μm, 15 min); the number of dead and dying cells was scored 8 h later (t = 24 + 8). Arrowheads denote the location of nuclei from healthy cells; arrows indicate apoptotic cells with condensed or fragmented nuclei. D, In the absence of pretreatment with synaptic activity, relatively high numbers (∼40%) of apoptotic cells were observed. Pretreatment with synaptic activity significantly (*p < 0.05) reduced the apoptotic effects of NMDA. Control data are from cells that were stimulated neither with synaptic activity nor with NMDA. Numbers above each bar indicate the number of cells assayed. E, The neuroprotective effects of synaptic activity also were monitored via LDH release. Cell stimulation was performed as described in C. *Significant difference (p < 0.05) from the NMDA treatment condition. NMDA administration was performed in media containing TTX (1 μm).