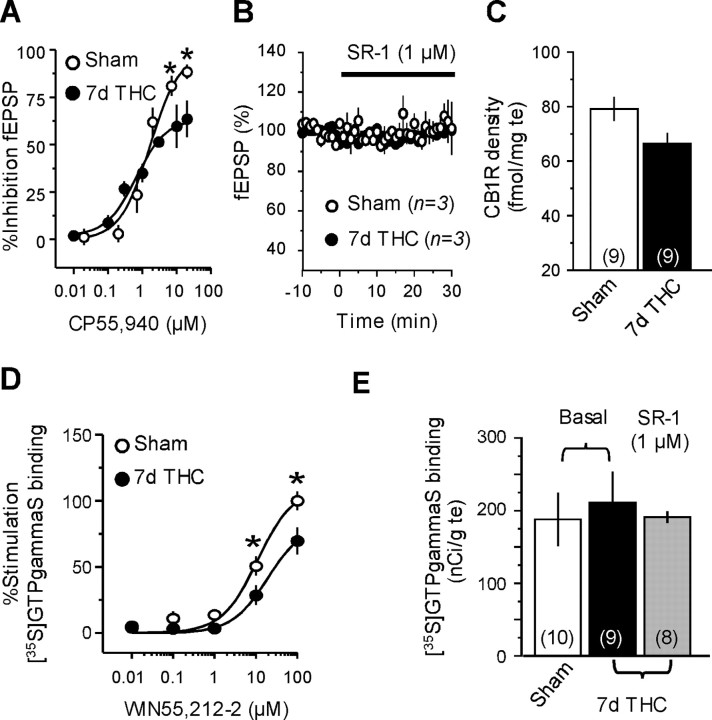
Figure 2.
Repeated THC exposure desensitizes CB1R in the NAc. A, Dose–response curves showing the inhibition of the fEPSP amplitude by the cannabinoid agonist CP55,940 in the NAc of mice repeatedly exposed to THC (n = 3–5) or vehicle (n = 3–5). Reduced CP55,940-mediated inhibition of accumbens fEPSP was observed in THC-treated mice (*p < 0.05). B, Perfusion of SR141716A (SR-1; 1μm) did not affect baseline synaptic transmission in the NAc of THC-treated (black circles; n = 3) or vehicle-treated (white circles; n = 3) mice, indicating the absence of residual THC prebound to CB1R. C, CB1R autoradiographic labeling in the accumbens after repeated exposure to THC or vehicle. D, Stimulation of [35S]GTPγS binding by the cannabinoid agonist WIN55,212-2 in the NAc of mice treated with THC (n = 9) or vehicle (n = 10) for 7 d. Impaired ability of the cannabinoid agonist to stimulate [35S]GTPγS binding was observed after repeated exposure to THC (*p<0.05).E, Absence of residual THC in brain sections after 7 d exposure. Similar basal [35S]GTPγS binding levels were measured in the NAc of sham (white bar; n = 10) and 7 d THC-treated (black bar; n = 9) mice, and incubation with the CB1R antagonist SR-1 (1 μm) did not increase basal [35S] GTPγS binding in the NAc of THC-injected mice (gray bar; n = 8).