Figure 3.
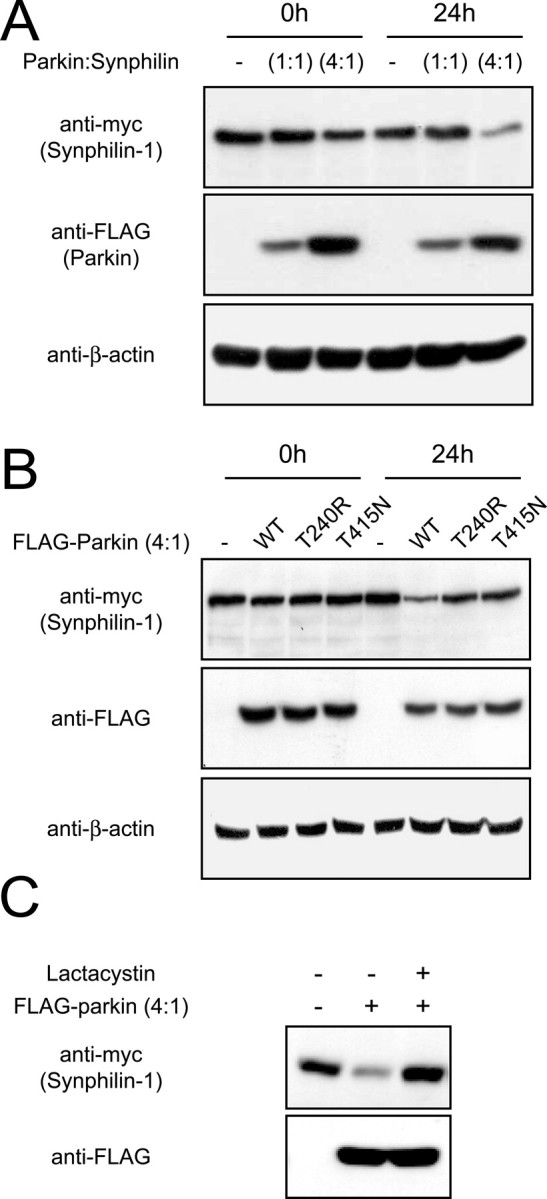
Degradation of synphilin-1 by parkin is dependent on their relative expression levels and on parkin catalytic competency. A, Anti-myc and anti-FLAG immunoblots of total cell lysates prepared from HEK 293 cells transfected with myc-tagged synphilin-1 without or with FLAG-tagged parkin at different cotransfection ratios (as indicated) and treated 24 h later with puromycin (100 μm) for 24 h. B, Anti-myc and anti-FLAG immunoblots of total cell lysates prepared from HEK 293 cells transfected with myc-tagged synphilin-1 without or with FLAG-tagged, wild-type parkin, or familial-PD parkin mutants, at a parkin/synphilin-1 cDNA cotransfection ratio of 4:1 and treated with puromycin (100 μm) for 24 h. In both cases, equal loading of the different cell lysates was verified by anti-β-actin immunoblotting. C, HEK 293 cells transfected with the indicated combinations of myc-tagged synphilin-1 and FLAG-tagged parkin were left untreated or treated with 5 μm clasto-lactacytstin β-lactone (lactacystin) for 16 h. Lysates prepared from these cells were immunoblotted with anti-myc or anti-FLAG as indicated. These experiments were duplicated with similar results.
