Figure 1.
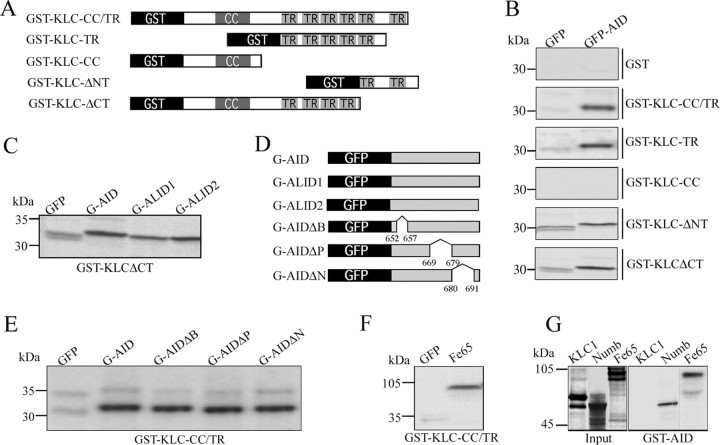
Nonspecific binding of AID and GST-KLC1 fusion proteins containing TRs. A, Schematic representation of GST-KLC1 deletion mutants used in GSTpull-down assays. For details, see Materials and Methods. B, Pull-down assays show that all GST-KLC constructs containing two or more TRs bind to in vitro-translated GFP-AID constructs and with lower affinity to the GFP-only control. Neither GST alone nor GST-KLC-CC (both lacking TRs) pulled down GFP-AID or GFP. The fact that TR-containing constructs pulled down both GFP-AID and to a lower extent the highly soluble protein GFP suggests that these are nonspecific protein-protein interactions. C, In addition to GFP-AID, both GFP-ALID1 and GFP-ALID2 were retained to a very similar extent on GST-KLCΔCT. Identical results were obtained with GST-KLC-TR or GST-KLC-CC/TR beads (data not shown). D, Schematic representation of GFP-APP intracellular domain deletion mutants used in GST pull downs (see Materials and Methods). E, GST-KLC-CC/TR binds with similar affinities to GFP-AID and GFP-AID constructs with deletions of the BaSS (G-AIDΔB), PEER (G-AIDΔP), or NPTY (G-AIDΔN) domains of APP. These represent domains that have been shown previously to affect trafficking of APP and map to the domain proposed by Kamal et al. (2000) to bind TR domain in KLC1. F, Beads containing GST-KLC with TR domains also pull down recombinant Fe65 with high affinity, again suggesting nonspecific binding of TR domains to proteins in this assay system. G, GST pull downs with GST fused to the intracellular domain of APP demonstrated binding to the known APP interacting proteins Fe65 and Numb, whereas no binding of KLC1 was observed under identical conditions.