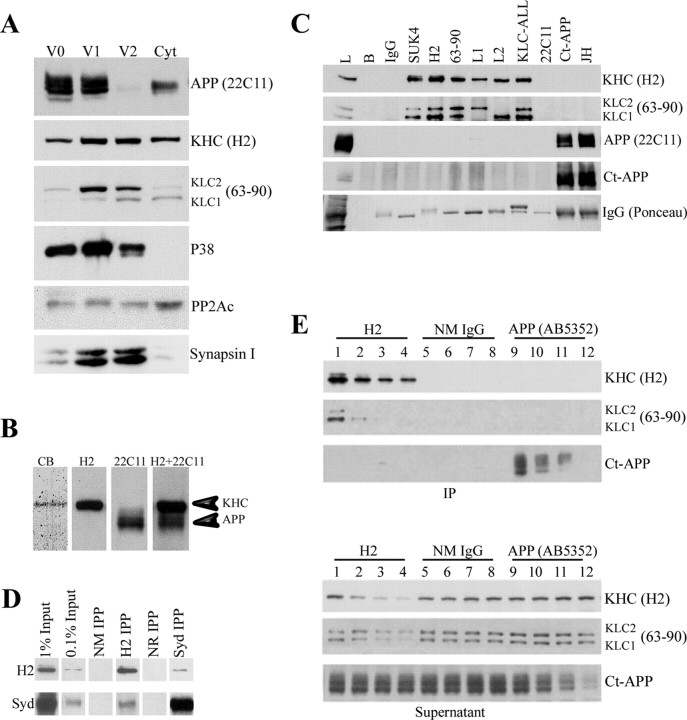
Figure 2.
Kinesin-1 and APP do not cofractionate or coimmunoprecipitate from brain lysate. A, Subcellular fractionation of mouse brain was performed as described previously (Morfini et al., 2002) to generate three vesicle fractions (V0, V1, and V2) and a cytosolic fraction (Cyt). Under the conditions of fractionation, KHCs and KLCs are found in all fractions, but KLCs exhibit differences in stoichiometry and isoform composition. In contrast, APP immunoreactivity is associated primarily with V0 and V1 fractions, with only trace amounts in V2 and low levels in the cytosol. V2 contains high levels of KHC, KLC1, KLC2, synaptophysin (P38), and synapsin I. This suggests that kinesin-1 and APP do not cofractionate in mouse brain. B, Immunoprecipitates of kinesin-1 from mouse brain show that Coomassie blue-stained levels of kinesin-1 heavy chain are obtained (CB), but no band corresponding to APP was seen. Immunoblots of whole-brain lysate show that kinesin-1 heavy chain (H2) is readily resolved from APP (22C11) in this gel system. These are distinguishable even when a mixture of both antibodies (H2+22C11) is used for immunoblotting. C, Immunoprecipitation of kinesin-1 from mouse brain using antibodies against either kinesin-1 heavy chains (SUK4 or H2) or light chains (63-90, L1, L2, or KLC-All) precipitated both KHC and KLC subunits (Pfister et al., 1989; Stenoien and Brady, 1997). Nevertheless, APP could not be detected in immunoprecipitates using kinesin-1 antibodies. Brain lysate (L) was a positive control. Negative controls, i.e., protein G beads without primary antibodies (B) or with normal mouse IgG show immunoprecipitation specificity. Similarly, kinesin-1 subunits were not detectable in immunoprecipitates with APP antibodies [Ct-APP and JH; see KHC (H2) for kinesin-1 heavy chain, KLC (63-90) for kinesin-1 light chain]. Antigens in immunoprecipitates were visualized using specific antibodies against kinesin-1 subunits (H2 or 63-90) or APP (22C11 or Ct-APP). D, Immunoblot analysis showed detectable amounts of the scaffolding protein JIP3/SYD in kinesin-1 immunoprecipitates (H2 IPP). Conversely, anti-JIP3 antibodies (SYd IPP) coprecipitate small but detectable amounts of KHC. Immunoprecipitations with normal mouse (NM IPP) or normal rabbit (NR IPP) IgGs were used as negative controls. Note the relative levels of JIP3/SYD compared with original protein input. E, Immunodepletion of kinesin-1 and APP from mouse brain lysate exhibits different depletion patterns. Immunodepletion of KHC from lysate with H2 comparing IPs and corresponding supernatant samples shows a gradual reduction in the level of both KHC (H2) and KLC (63-90) in the pellet (lanes 1-4) and the supernatant (lanes 13-16) after depletion cycles. In contrast, no change in APP level could be detected (Ct-APP, lanes 1-4 and 13-16, respectively). Similarly, a reduction in APP level was observed after depletion using APP-specific antibodies AB5352 (Ct-APP, pellet, lanes 9-12; supernatant, lanes 21-24), but no change in the level of kinesin-1 could be detected (KHC, lanes 9-12, 21-24; KLC, lanes 9-12, 21-24).