Abstract
Electron capture dissociation (ECD), which generally preserves the position of labile post-translational modifications, can be a powerful method for de novo sequencing of proteins and peptides. In this report, ECD product-ion mass spectra of singly and doubly sodiated, nonphosphorylated, and phosphorylated peptides are presented and compared with the ECD mass spectra of their protonated counterparts. ECD of doubly charged, singly sodiated peptides yielded essentially the same sequence information as was produced by the corresponding doubly protonated peptides. The presence of several sodium binding sites on the polypeptide backbone, however, resulted in more complicated spectra. This situation is aggravated by the zwitterionic equilibrium of the free acid peptide precursors. The product-ion spectra of doubly and triply charged peptides possessing two sodium ions were further complicated by the existence of isomers created by the differential distribution of sodium binding sites. Triply charged, phosphorylated precursors containing one sodium, wherein the sodium is attached exclusively to the PO4 group, were found to be as useful for sequence analysis as the fully protonated species. Although sodium adducts are generally minimized during sample preparation, it appears that they can nonetheless provide useful sequence information. Additionally, they enable straightforward identification of a peptide’s charge state, even on low-resolution instruments. The experiments were carried out using a radio frequency-free electromagnetostatic cell retrofitted into the collision-induced dissociation (CID) section of a hybrid quadrupole/time-of-flight tandem mass spectrometer.
Keywords: Electron capture dissociation, Sodium-adducted peptides
Graphical abstract
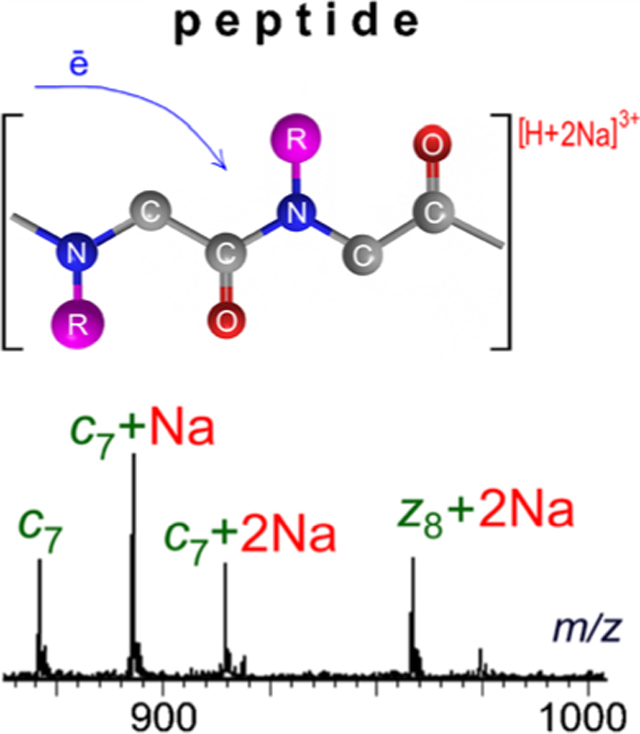
Introduction
The presence of alkali-metal cations such as Na+ or K+ strongly influences electrospray ionization [1–3]. An excess of such cations with high affinities for charged analytes can suppress formation of protonated analyte signals. Desalting on a column is commonly performed to reduce the concentration of cations, and addition of formic or acetic acid is routinely used to enhance protonation over metal-ion adduction. Despite such steps, sodium and potassium can remain tightly bound to certain analytes. Measurement of the shift in m/z of a sodiated molecular ion peak can, in some instances, provide a simple means for determining the charge state of an analyte ion [4, 5].
Samples isolated from biological sources commonly contain significant concentrations of sodium, such that sodiated species are routinely observed in the electrospray spectra. Hudgins et al. examined ECD of natural and synthetic peptides doubly and triply charged with alkali cations (“alkalized”) instead of protons, of peptides with fixed charges, and of peptides devoid of labile hydrogens [6]. The product-ion spectra of peptides without acidic residues that promote proton migration displayed mass peaks corresponding, respectively, to the alkalized analogs of the c′-type and z•-type ions produced from their protonated analogs. ECD of a disulfide-linked dimer also produced fragments because of disulfide cleavage as well as the alkalized analogs of c′-and z•-type ions. lavarone et al. investigated ECD of a lysine-and alanine-containing peptide dialkalized with different combinations of H+, Li+, and Cs+ [7]. ECD of (M + 2Li)2+ and (M + 2Cs)2+ precursors was found to produce mono-and dialkalized analogs of the same c′ -and z• type ions observed in the product-ion spectrum of (M + 2H)2+; however, ECD of (M + H + Li)2+ resulted exclusively in lithiated c′-and z•-type ions, indicating a preference for neutralization and cleavage at protonated sites over alkalized sites. The abundance of a dialkalized fragment ion was observed to increase with its mass, and the abundances of cesiated fragment ions formed from ECD of [M + Cs + Li]2+ were on average an order of magnitude greater than those of the corresponding lithiated fragment ions. In independent reports on electron-induced dissociation (which encompasses ECD) of peptides, Sargaeva et al. showed product-ion mass spectra of sodiated and potassiated peptides [8], whereas Enyenihi showed product-ion mass spectra of sodiated peptides [9]; all spectra contained mass peaks corresponding to the alkalized precursors minus informative side chain losses as well as peptide back-bone cleavages resulting in nonalkalated a-, b-, c-, x-, y-, and z-type fragment ions. In the only other report that could be found involving ECD of sodiated peptide-like molecules, Bogdanov et al. examined the fragmentation patterns of a group of mixed protonated-sodiated peptoids, which are peptide-mimicking oligomers based on the structure of poly(N-substituted glycine) and resistant to protease digestion [10]. Backbone fragmentation of these peptoids occurred primarily at N–Cα bonds regardless of the type of charge carrier. Peaks corresponding to sodiated fragment ions from the N-terminus (i.e., c′-type fragments), were universally present in the product-ion spectra of all peptoids; however, the appearance of mass peaks corresponding to sodiated fragment ions from the C-terminus (i.e., z•-type fragments) varied considerably depending on the type of charge carrier.
The effects of adduction by nonalkali metals on ECD of peptides have been studied in a few cases. Fung et al. demonstrated that useful ECD product-ion mass spectra of these metalated peptides could be generated from peptides cationized with the divalent alkaline-earths Mg2+, Ca2+, Sr2+, and Ba2+, and furthermore, that the appearance of those spectra differed very little from one another [11]. It was also found in this study that the ECD product-ion mass spectra of the metalated peptides exhibited enhanced signals corresponding to minor species such as (c – 1)+• and (z +1)+ ions. Liu and Håkansson compared ECD of doubly protonated Tyr2-sulfated cholecystokinin and Tyr12-sulfated gastrin II with ECD of those two peptides doubly charged with Ca2+, Mn2+, Zn2+, and Fe2+, and ECD of Tyr12-sulfated gastrin II triply charged in the form [M + 2Ca – H]3+ [12]. Contrary to what is typically observed, ECD of the doubly protonated precursors of the two peptides resulted in complete loss of SO3 from all product ions and, thus, precluded localization of the sulfate groups. By contrast, ECD of all of the metalated forms of the peptides produced sulfated c′-and z•-type product ions with high sequence coverage and, thereby, allowed sequencing and sulfate localization. In a related study, Liu and Håkansson investigated ECD of amidated Substance P in the triply charged form [M + H + divalent metal]3+ (where metal = Mg-Ba, Mn, Fe, Co, Ni, Cu, Zn, and Mn-Zn) [13]. The signal strengths of the fragment ions varied widely among the production mass spectra suggesting that the mechanisms for ECD were strongly dependent on the choice of divalent metal ion.
For more than decade, the only commercial access to ECD remained on Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometers. In very recent years, two new approaches based on hybrid Q-TOF instruments have been reported. A high-throughput ECD was realized by adding a branched radio frequency ion trap on TrippleTOF-5600 Q-TOF mass spectrometer (SCIEX, Toronto, ON. Canada) [14], and atmospheric pressure electron capture dissociation (AP-ECD) was realized by fitting an AP-ECD source to a Xevo G2-S Q-TOF mass spectrometer (Waters Co., Milford, MA, U.S.A.) [15].
In 2008, the present authors described an electromagnetostatic (EMS) cell that can be retrofitted into virtually any existing type of mass spectrometer [16]. In this report, the EMS cell retrofitted into a hybrid Q-TOF tandem mass spectrometer was used to investigate the effects of sodium adducts on the ECD behavior of peptides with the aim of examining ECD’s potential for characterizing peptides with sodium adducts. Results of ECD of monosodiated and disodiated forms of doubly charged cations of Substance P in its amidated and free acid forms and of doubly and triply charged cations of phosphorylated tyrosine kinase peptide 3 in its amidated form are presented and discussed.
Experimental
Materials and Sample Preparation
Authentic standards for Substance P [free acid – Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-OH, monoisotopic MW = 1348.6 Da; amidated – Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2, monoisotopic MW = 1347.6 Da] and for amidated phosphorylated tyrosine kinase peptide 3 [Arg-Arg-Leu-Ile-Glu-Asp-Ala-Glu-Tyr(PO3H2)-Ala-Ala-Arg-Gly-NH2, monoisotopic MW = 1597.8 Da] were purchased from American Peptide Co. (Sunnyvale, CA, USA) and were used without further purification. Each of the peptide standards was dissolved in a solution of 50% water/50% methanol. Sodium chloride (NaCl) was added to each of the standard solutions of Substance P to bring the sodium ion concentration to 0.25 mM; sodium acetate (NaOAc) was added to the standard solution of phosphorylated tyrosine kinase peptide 3 to bring the sodium ion concentration to 1.5 mM.
Mass Spectrometry
All mass spectrometry was performed using a QSTAR XL electrospray ionization (ESI), hybrid quadrupole/time-of-flight (Q-TOF) mass spectrometer system (SCIEX, Concord, ON, Canada). The QSTAR was modified by shortening the original radio-frequency, quadrupole, CID cell (Q2) by 76 mm and inserting a radio-frequency-free EMS ECD cell assembly into the space created between the first quadrupole (Q1, Figure 1) and the shortened CID cell (Q2, Figure 1). Compared with the EMS cell used in a triple quadrupole (QqQ) mass spectrometer system (Agilent G6400; Agilent Technologies, Santa Clara, CA, USA) for recording ECD spectra of phosphorylated peptides [17], the design of the QSTAR XL cell was simplified by replacing the electromagnet with a permanent disc magnet. Thus, the QSTAR EMS cell assembly comprised, from entrance-to-exit order, a four-electrode electrostatic lens, a permanent magnet, a heated filament within a holder that also served as an electrostatic lens, a permanent magnet (Chino Magnetism, Fairfield, NJ, USA), and a four-electrode electrostatic lens. Both of the permanent magnets were axially polarized, Sm2Co17 rings (25.4 mm diameter, 1.0 mm thick, 3.0 mm bore) that could withstand temperatures up to 250°C. The polarities of the magnets were aligned on opposite sides of the filament assembly to create “magnetic bottles” [18, 19] for trapping electrons emitted from the heated filament (insert, Figure 1). The filament, which served as the ECD cell’s source of electrons, was a loop (1.0 mm diameter) of tungsten-rhenium wire as described in previous reports [16, 17, 20, 21]. For this study, potentials for the filament and permanent magnet were each set at +15 V, for the first electrostatic lens at −15 V, and for the last electrostatic lens at +10 V.
Fig. 1.
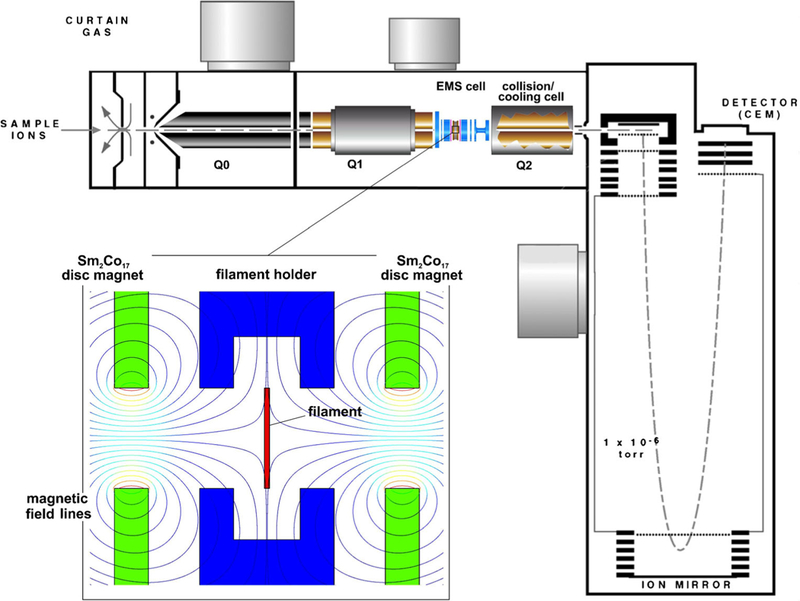
Schematic of the QSTAR XL mass spectrometer system with an RF-free EMS ECD cell retrofitted into the CID/collisional cooling quadrupole section of the instrument. The lower left insert shows an enlarged cross-sectional view of the EMS ECD cell’s interior
Each peptide solution was electrosprayed at a flow rate of 10 μL/min. The doubly protonated ([M + 2H]2+), singly sodiated ([M + H + Na]2+), and multiply sodiated ([M + nNa]2+, n ≥ 2) species were selected as precursor ions by the first quadrupole mass analyzer (Q1, Figure 1) and transferred to the EMS ECD cell to merge with low energy electrons. Ions emerging from the EMS cell passed through the shortened CID cell (Q2, Figure 1), which was operated in a transmission mode only, and on into the time-of-flight mass analyzer.
Results and Discussion
Amidated Substance P is commonly used as a standard for evaluating ECD efficiency [22–26]. ECD product-ion mass spectra of amidated Substance P produced with the modified QSTAR used in this study exhibited the well-known ECD pattern of c-fragmentation with an efficiency approaching 10% (Figure 2a; Table S1 in Supplemental Materials). Compared with the previously reported ECD efficiency obtained with the electromagnet-based EMS cell that was retrofitted into the Agilent G6460 QqQ instrument [27], the ECD efficiency of the QSTAR cell was about five times greater. Further magnetic field and emitter refinements should lead to yet higher efficiencies.
Fig. 2.
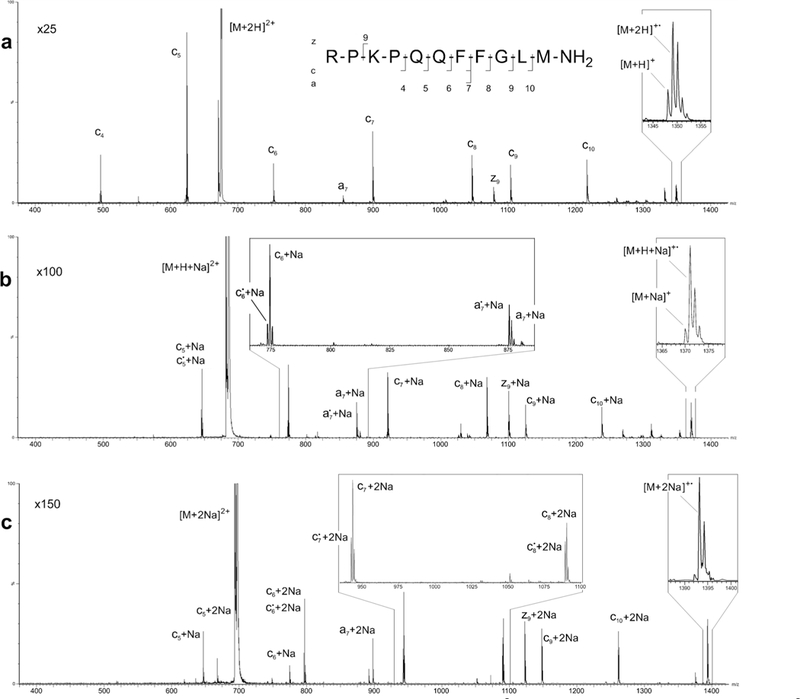
ECD product-ion mass spectra of the (a) doubly protonated precursor [M + 2H]2+, (b) singly sodiated precursor [M + H + Na]2, and c doubly sodiated precursor [M + 2Na]2+ of amidated Substance P
ECD of the [M + H + Na]2+ and [M + 2Na]2+ precursors of amidated Substance P (Figure 2b, c; Table S1 in Supplemental Materials) produced almost exclusively monosodium and disodium analogues (i.e., nonprotonated fragments) of the c5–10, a7, and z9 fragment ions observed in the product-ion spectrum of the [M + 2H]2+ precursor (Figure 2a; Table S1 in Supplemental Materials). No sodium analogs of the c4 fragment seen in the [M + 2H]2+ spectrum were observed in the [M + H + Na]2+ and [M + 2Na]2+ spectra. Satellite peaks corresponding to hydrogen loss from some of the metal-bound fragments [e.g., c5• + Na, c6• + Na and a7• + Na (Figure 2b, insert) and c6• + 2Na, c7• +2Na and c8• + 2Na (Figure 2c, insert)] are also present in the ECD product-ion spectra. As reported in an earlier study,7 the relative abundances of the sodium-adducted fragment ions for the most part increased with fragment-ion mass. Exceptions to this trend are observed in the cases of c7 and c9, which is probably tied to the increased relative intensities observed for the a7 and z9 product ions.
Formation of the c5 + 2Na and c6 + 2Na fragments (Figure 2c, Table S1 in Supplemental Materials) places the sites of sodium binding on two of the peptide’s first five residues, which is in accordance with the general view that the sites of protonation in doubly protonated Substance P are Arg1 and Lys3 [25, 28]. Formation of the z9 + 2Na fragment, however, indicates that when one of two sodium atoms is attached to Lys3, the second sodium ion resides on one of the remaining 4–11 residues. This seemingly indicates that sodium adduction produces different positional isomers. The production spectrum produced from the [M + 2Na]2+ precursor (Figure 2c) indicates that similar sequence information results from the different sodium-bound isomers.
The ECD spectra produced from the free-acid form of Substance P (Figure 3; Table S2 in Supplemental Materials) are distinct in several respects from those produced by the amidated form (Figure 2; Table S1 in Supplemental Materials). In addition to the nearly complete series of c-type fragments produced by ECD of both forms of Substance P, ECD of the protonated free-acid (Figure 3a, Table S2 in Supplemental Materials) also produces a nearly complete series of a-type fragments—only a4 is missing, and z9 is observed in place of a9. Similar ECD mass spectra were recorded by the present authors on a Thermo Finnigan 7 T LTQ FT Ultra linear ion trap mass spectrometer system (Figure S1, Supplementary Materials). In an earlier study, Marshall and coworkers reported nearly identical ECD product-ion mass spectra via FT ICR mass spectrometry [29, 30]. These investigators posited that conversion of amidated Substance P to the free acid form removed the C-terminal basic site and, thus, increased the likelihood of formation of a zwitterionic structure that promoted ECD of the free-acid form into a-type fragment ions. Hence, the appearance of the a-type fragment series in the ECD spectrum recorded in the present study (Figure 3a, Table S2 in Supplemental Materials) provides yet further evidence of a positive charge shift toward the N-terminus and a zwitterionic charge distribution in the doubly protonated free-acid form of Substance P. The fact that the a-type fragments are even-electron a’ species [31] lends additional credence to this idea of facilitated proton migration.
Fig. 3.
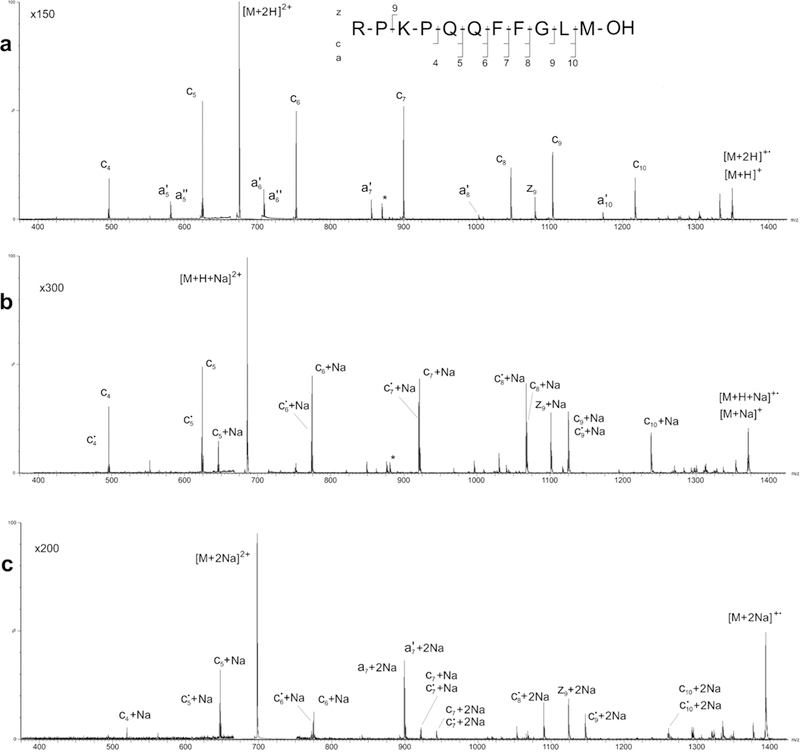
ECD product-ion mass spectra of the (a) doubly protonated precursor [M + 2H]2+, (b) singly sodiated precursor [M + H + Na]2+, and c doubly sodiated precursor [M + 2Na]2+ of the free acid form of Substance P
As a side note, it is interesting that ECD of both forms of Substance P in a much earlier report [32] resulted in product-ion mass spectra that were essentially indistinguishable. The apparent lack of difference between ECD of the amidated and free-acid forms exhibited in this previous paper was most likely the result of the ECD product-ion spectrum of the free-acid form being recorded, as was noted in the paper, with a much lower signal-to-noise ratio.
As in the case of the amidated form, the ECD product-ion spectrum of the [M + H + Na]2+ precursor of the free-acid form of Substance P (Figure 3b; Table S2 in Supplemental Materials) exhibits almost exclusively singly adducted analogs of the c5–10 and z9 fragment ions observed in the spectrum of the [M + 2H]2+ precursor. Signals corresponding to protonated c4 and c5 fragments were also present in the free-acid spectrum, and formation of the even-electron satellite products (i.e., c•x + Na ions), was more prevalent (Figure 3b, c; Table S2 in Supplemental Materials) than that evident in the corresponding spectrum (Figure 2b, c; Table S1 in Supplemental Materials) of amidated Substance P. The most pronounced effect of replacing one of doubly protonated Substance P’s protons with a sodium ion is total suppression of the production of a-type ECD fragments. If Na+ is attached to the C-terminal oxygen, which is presumably the preferred location in the free-acid form of Substance P, considerable alteration in the zwitterionic property observed in the nonadducted peptide would be expected, and loss of a-type fragment production as observed in this study would be a logical consequence.
Replacing the proton in the [M + H + Na]2+ precursor of Substance P’s free-acid form with a sodium ion resulted in an ECD product-ion spectrum (Figure 3c; Table S2 in Supplemental Materials) that was markedly different from that of its amidated [M + 2Na]2+ counterpart (Figure 2c; Table S1 in Supplemental Materials). Specifically, the regularity in mass-shift between the fragments is broken (i.e., there was a single sodium attached exclusively to the c4 and c6 fragments, two sodium ions attached exclusively to the c5, c8-c10, a7, and z9 fragments, and both one and two sodiums attached to the c7 fragment). In addition, the a7 fragment became the most abundant fragment in the product-ion spectrum. ECD product-ion spectra of the free-acid form of Substance P carrying three or more sodium adducts contained very little analytically useful information. Specifically, the ECD product-ion spectrum of [M + 3Na – H]2+ (Figure S2A, Supplementary Materials) only exhibits signals for a7 and z9, and that of [M + 4Na – 2H]2+ (Figure S2B, Supplementary Materials) only shows signals for c8 and c9.
In an earlier study,17 the present authors found that phosphorylated tyrosine kinase peptide 3 (Pp1598) does not dissociate easily under the relatively high efficiency ECD conditions that typically prevail in FT ICR instruments. This refractory behavior toward ECD dissociation was speculatively attributed to the formation of salt-bridges [33] between the phosphogroup and a protonated arginine that would hinder dissociation of ionneutral c/z-complexes produced by the N–Cα cleavages of this peptide. By contrast, it was found in the same study17 that at a total electron capture efficiency comparable to that observed in the FT ICR instruments, the extent of ECD of Pp1598 in an EMS ECD cell retrofitted into an Agilent 6460 triple quadrupole (Agilent Technologies) was significantly greater. It was postulated that thermal radiation emitted by the ECD cell’s hot filament (electron source) provided sufficient energy to break apart the salt-bridges holding the c/z-complexes together. It was decided in the present study to investigate the effect of sodium adduction on this interesting phenomenon.
The clean ECD product-ion mass spectrum of doubly protonated Pp1598 recorded with the modified SCIEX Q-TOF mass spectrometer (Figure 4a) exhibited uniform spacing of fragments, and very closely matched the fragmentation pattern and efficiency of the spectrum recorded earlier with the modified Agilent triple quadrupole (only the low mass c2 and c4 fragments were missing in the latter).17 Hence, the ECD conditions in the two cells must have been nearly identical despite their differing geometries, an inference that supports the idea that salt-bridged ion-neutral c/z-complexes formed during ECD absorb radiation from the EMS cell’s hot electron emitters and, further, that this extra energy breaks apart the complexes.
Fig. 4.
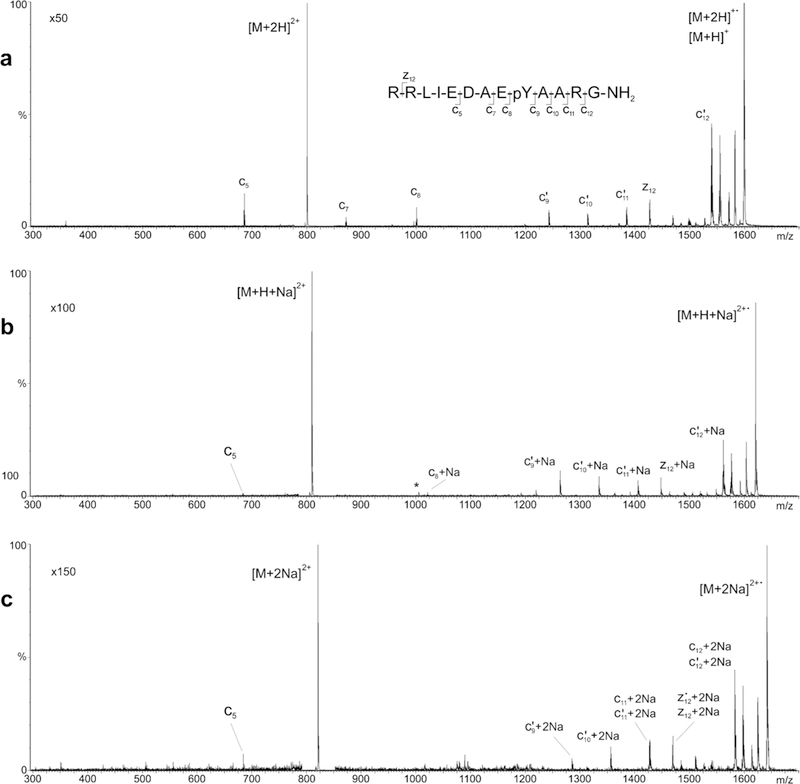
ECD product-ion mass spectra of the (a) doubly protonated precursor [M + 2H]2+, (b) singly sodiated precursor [M + H + Na]2+, and c doubly sodiated precursor [M + 2Na]2+ of phosphorylated tyrosine kinase peptide 3
The ECD product-ion spectra of the singly sodiated ([M + H + Na]2+) and doubly sodiated ([M + 2Na]2+) precursors of Pp1598 consist exclusively of mass peaks corresponding, respectively, to mono-adducted (Figure 4b) and di-adducted (Figure 4c) analogues of the c9–12 and z12 fragmention signals observed in the ECD product-ion spectrum of Pp1598’s [M + 2H]2+ precursor. Peaks corresponding to the low-mass c5–8 fragments are almost completely absent from the sodiated spectra. This alteration in the pattern of Pp1598’s fragmentation as a result of sodium adduction contrasts with the nearly unchanged pattern of Substance P’s fragmentation following sodium adduction. Clearly, fragmentation from Pp1598’s N-terminus effectively stops where the phosphogroup is attached. This observation is reasonable as the electronegative character of the phosphogroup’s oxygen makes it a preferred site for sodium attachment and, once attached, the sodium’s charge precludes formation of any smaller charged fragments.
The sequencing efficiency of ECD can be quite high for triply protonated peptide precursors.33 This has been convincingly demonstrated for Pp1598 in an earlier study by the present authors17 using triple quadrupole and FT ICR mass spectrometers. The ECD product-ion mass spectrum of triply protonated Pp1598 (Figure 5a) recorded with the QSTAR used in the present study is qualitatively almost identical to those earlier spectra. In all of the preceding cases, the production spectrum of triply protonated Pp1598 provides 100% coverage of the peptide’s primary structure; the coverage is even redundant because of the substantial overlap of the complimentary c-and z-type of fragments (Figure 5a).
Fig. 5.
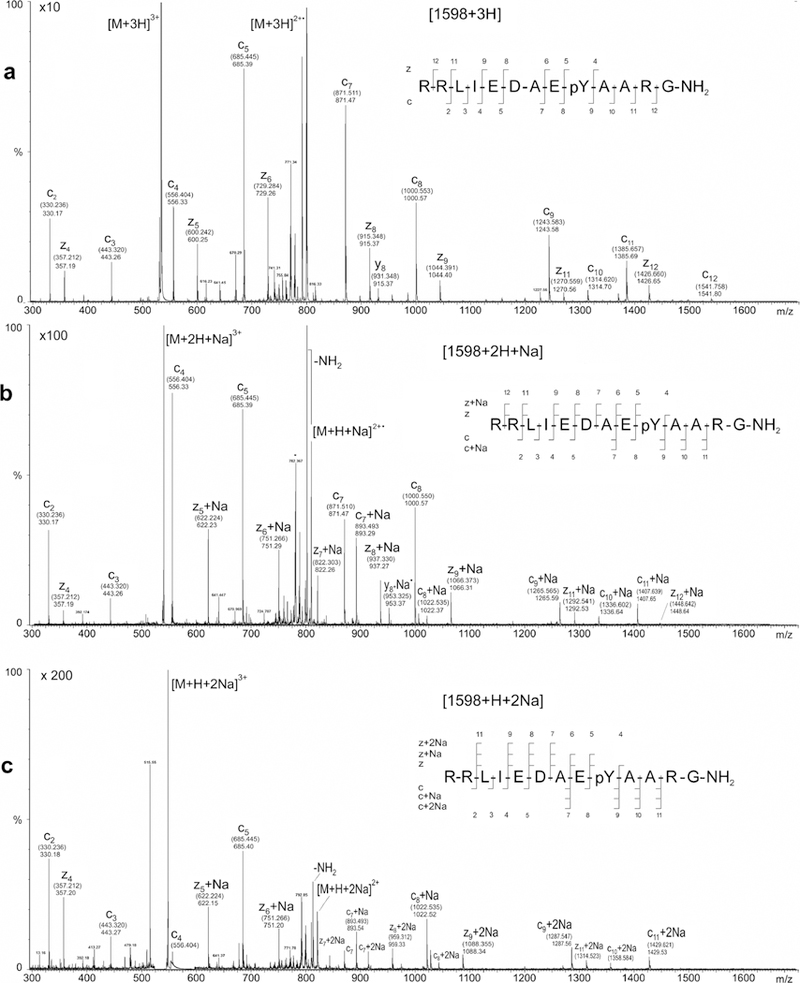
ECD production mass spectra of the (a) triply protonated precursor [M + 3H]3+, (b) singly sodiated precursor [M + 2H + Na]3+, and (c) doubly sodiated precursor [M + H + 2Na]3+ of phosphorylated tyrosine kinase peptide 3. Theoretical m/z values for assigned fragment-ion peaks are enclosed in parentheses; measured m/z values are shown without parentheses
Obviously, the spectrum obtained from the triply protonated precursor of Pp1598 ([M + 3H]3+) is substantially more informative than that obtained with the doubly protonated precursor (Figure 4b). None of the informational content in the production spectrum of triply protonated Pp1598 is lost when one of the three protons is replaced with sodium. Except for the disappearance of the c12 fragment, the ECD product-ion spectrum of triply charged Pp1598 with a single sodium adduct ([M + 2H + Na]3+, Figure 5b) is qualitatively the same as that of the [M + 3H]3+ precursor. The m/z-ratios of the sodium-containing fragments (viz., c9–11, z5–9, z11, and z12) are nominally shifted relative to the corresponding peaks in the ECD spectrum of [M + 3H]3+ by 22 (i.e., the mass of sodium minus one), whereas the m/z-ratios of the nonsodium-containing fragments (viz., c2–5 and z4) are not shifted. The shift in the peaks corresponding to c9 and z5 and the nonshift in the peaks corresponding to c8 and z4 unambiguously locate the site of sodium attachment on the phosphogroup.
In general, the informational content of the ECD product-ion spectrum of triply charged Pp1598 with two sodium adducts ([M + H + 2Na]3+, Figure 5c) is qualitatively similar to that of the [M + 2H + Na]3+ precursor, but the simultaneous presence of singly and doubly sodiated fragments makes interpretation of the former more complicated than of the latter. Signals for all of the fragments, except c12 and z12, observed in the ECD production spectra of the [M + 3H]3+ and [M + 2H + Na]3+ precursors of Pp1598 are also present in the ECD product-ion spectra of the [M + H + 2Na]3+. Hence, the site of phosphorylation can still be easily and unambiguously identified. The absence of a mass peak for sodiated z4 and the presence of peaks for singly sodiated z5 and z6 indicate that there are no sites of sodium attachment on the C-terminal side ofPp1598’s phosphorylated Tyr9. The appearance of signals corresponding to doubly sodiated forms of c7–8 and z7–8 clearly points to multiple sites of sodium attachment on the N-terminal side ofPp1598’s phosphorylated Tyr9. This finding indicates in turn that Pp1598’s [M + H + 2Na]3+ precursor or its reduced form [M + H + 2Na]2+• exists as a distribution of doubly sodiated isomers.
Conclusion
Altogether, the singly sodiated precursors of both the amidated and free-acid forms of Substance P and the doubly sodiated precursor of the amidated form yield sequence information very close in content to that yielded by their doubly protonated precursors. By contrast, the doubly sodiated precursor of Substance P’s free-acid form yields a complex ECD production spectrum, which presumably results from alteration in the peptide’s zwitterionic character, that is much less useful for structural analysis than that of its protonated counterparts. The ECD product-ion spectrum of free-acid Substance P adducted with more than two sodiums does not contain enough structural information for analytical purposes.
The site of phosphorylation is preserved during ECD of singly and doubly sodiated forms of doubly charged, amidated phosphorylated tyrosine kinase peptide 3 (Pp1598). For practical purposes, backbone cleavage is limited to the amino acid chain between the phosphorylated Tyr9 and the C-terminus. This suggests that similarly phosphorylated peptides would likewise not be completely sequenced by ECD of their doubly charged precursors.
ECD of triply protonated Pp1598 both preserves the site of phosphorylation and nearly yields the entire amino acid sequence of the peptide. Replacing a single proton with sodium results in a product-ion spectrum that is as simple as that of the fully protonated precursor. Replacing two of the protons with sodium leads to an ECD product-ion spectrum that is complicated because of the existence of Na-positional isomers. In general, it appears that ECD MS/MS can be performed on sodium containing samples of peptides without undue effort to remove the sodium.
Supplementary Material
Acknowledgments
The principal source of support for this research was provided by the Bio-Organic Biomedical Mass Spectrometry Resource at UCSF (A.L. Burlingame, Director) supported by the Biomedical Technology Research Centers program of the NIH National Institute of General Medical Sciences, NIH NIGMS 8P41GM103481; other aspects of this research were supported by grants from the NSF (CHE-0924027), the Oregon Nanoscience and Microtechnologies Institute (no. 09-31 no. 3.5), NIH NCRR (R01RR026275), and NIH NIEHS (ES000210 -Environmental Health Sciences Center). The authors thank Larry M. Nelson (Department of Chemistry, Oregon State University, Corvallis, OR, USA) for his assistance in fabricating components of the EMS ECD cell used in this study, as well as Bruce Thompson and Igor Chernushevich (SCIEX, Concord, ON, Canada) for providing key components for the QSTAR XL, technical instructions, suggestions, and encouragement without which this project could not have been completed.
Footnotes
Electronic supplementary material The online version of this article (doi: 10.1007/s13361-015-1230-y) contains supplementary material, which is available to authorized users.
References
- 1.Van Berkel GJ, McLuckey SA, Glish GL: Preforming ions in solution via charge-transfer complexation for analysis by electrospray ionization mass spectrometry. Anal. Chem. 63, 2064–2068 (1991) [Google Scholar]
- 2.Van Berkel GJ, McLuckey SA, Glish GL: Electrochemical origin of radical cations observed in electrospray ionization mass spectra. Anal. Chem. 64, 1586–1593 (1992) [Google Scholar]
- 3.Iavarone AT, Udekwu OA, Williams ER: Buffer loading for counteracting metal salt-induced signal suppression in electrospray ionization. Anal. Chem. 76, 3944–3950 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neubauer G, Anderegg RJ: Identifying charge states of peptides in liquid chromatography/electrospray ionization mass spectrometry. Anal. Chem. 66, 1066–1061 (1994) [Google Scholar]
- 5.Senko MW, Beu SC, McLafferty FW: Mass and charge assignment for electrospray ions by cation adduction. J. Am. Soc. Mass Spectrom. 4, 828–830 (1993) [DOI] [PubMed] [Google Scholar]
- 6.Hudgins RR, Håkansson K, Quinn JP, Hendrickson CL, Marshall AG: Electron Capture Dissociation of Peptides and Proteins Does Not Require a Hydrogen Atom Mechanism. Proceedings of the 50th ASMS Conference on Mass Spectrometry and Allied Topics, Orlando, FL: (2002) [Google Scholar]
- 7.Iavarone AT, Paech K, Williams ER: Effects of charge state and cationizing agent on the electron capture dissociation of a peptide. Anal. Chem. 76, 2231–2238 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sargaeva NP, Lin C, O’Connor PB: Identification of aspartic and isoaspartic acid residues in amyloid β peptides, including Aβ1–42, using electron–ion reactions. Anal. Chem. 81, 9778–9786 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enyenihi AA: Tandem Mass Spectrometry of Biomolecules: Applications and New Methods Ph.D. Dissertation, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA: (2010) [Google Scholar]
- 10.Bogdanov B, Zhao X, Robinson DB, Ren J: Electron capture dissociation studies of the fragmentation patterns of doubly protonated and mixed protonated-sodiated peptoids. J. Am. Soc. Mass Spectrom. 25, 1202–1216(2014) [DOI] [PubMed] [Google Scholar]
- 11.Fung YME, Liu H, Chan T-WD: Electron capture dissociation of peptides metalated with alkaline-earth metal ions. J. Am. Soc. Mass Spectrom. 17, 757–771 (2006) [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Håkansson K: Electron capture dissociation of tyrosine o-sulfated peptides complexed with divalent metal cations. Anal. Chem. 78, 7570–7576 (2006) [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Håkansson K: Divalent metal ion–peptide interactions probed by electron capture dissociation of trications. J. Am. Soc. Mass Spectrom. 17, 1731–1741 (2006) [DOI] [PubMed] [Google Scholar]
- 14.Baba T, Campbell JL, Yves Le Blanc JC, Hager JW, Thomson BA: Electron capture dissociation in a branched radio-frequency ion trap. Anal. Chem. 87, 785–792 (2015) [DOI] [PubMed] [Google Scholar]
- 15.Robb DB, Brown JM, Morris M, Blades MW: Tandem mass spectrometry using the atmospheric pressure electron capture dissociation ion source. Anal. Chem. 86, 4439–4446 (2014) [DOI] [PubMed] [Google Scholar]
- 16.Voinov VG, Deinzer ML, Barofsky DF: Electron capture dissociation in a linear radiofrequency-free magnetic cell. Rapid Commun. Mass Spectrom. 22, 3087–3088 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voinov VG, Bennett SE, Beckman JS, Barofsky DF: ECD of tyrosine phosphorylation in a triple quadrupole mass spectrometer with a radio-frequency-free electromagnetostatic cell. J. Am. Soc. Mass Spectrom. 25, 1730–1738 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bittencourt JA: Fundamentals of Plasma Physics, 3rd edn, pp. 77–84. Springer, New York: (2004) [Google Scholar]
- 19.Chen FF: Introduction to Plasma Physics and Controlled Fusion, 2nd edn, pp. 30–49. Springer, New York: (2006) [Google Scholar]
- 20.Voinov VG, Beckman JS, Deinzer ML, Barofsky DF: Rapid Commun. Mass Spectrom. 23(18), 3028–3030 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voinov VG, Deinzer ML, Barofsky DF: Electron-capture dissociation (ECD), collision-induced dissociation (CID), and ECD/CID in a linear radiofTequency-free magnetic cell. Anal. Chem. 81, 1238–1243 (2009)19117494 [Google Scholar]
- 22.Axelsson J, Palmblad M, Håkansson K, Hakansson P: Electron capture dissociation of Substance P using a commercially available fourier transform ion cyclotron resonance mass spectrometer. Rapid Commun. Mass Spectrom. 13, 474–477 (1999) [DOI] [PubMed] [Google Scholar]
- 23.Håkansson K, Emmett MR, Hendrickson CL, Marshall AG: High-sensitivity electron capture dissociation tandem fticr mass spectrometry of microelectrosprayed peptides. Anal. Chem. 73, 3605–3610 (2001) [DOI] [PubMed] [Google Scholar]
- 24.Tsybin YO, Witt M, Baykut G, Kjeldsen F, Hakansson P: Combined infrared multiphoton dissociation and electron capture dissociation with a hollow electron beam in Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 17, 1759–1768 (2003) [DOI] [PubMed] [Google Scholar]
- 25.Mihalca R, Kleinnijenhuis AJ, McDonnell LA, Heck AJR, Heeren RMA: Electron capture dissociation at low temperatures reveals selective dissociations. J. Am. Soc. Mass Spectrom. 15, 1869–1873 (2004) [DOI] [PubMed] [Google Scholar]
- 26.Zubarev RA, Witt M, Baykut G: Twofold efficiency increase by selective excitation of ions for consecutive activation by ion–electron reactions and vibrational excitation in tandem Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 77, 2992–2996 (2005) [DOI] [PubMed] [Google Scholar]
- 27.Voinov VG, Lin Yu Chu, Bennett SE, Beckman JS, Barofsky DF: ECD in an RF-Free Electromagnetostatic Cell on a Triple Quadrupole Mass Spectrometer. Proceedings of the 60th ASMS Conference on Mass Spectrometry and Allied Topics, Vancouvser, Canada, 20–24 May (2012) [Google Scholar]
- 28.Gill AC, Jennings KR, Wyttenbach T, Bowers MT: Conformations of biopolymers in the gas phase: a new mass spectrometric method. Int. J. Mass Spectrom. 195/196, 685–697 (2000) [Google Scholar]
- 29.Emmett MR, Tsybin YO, Haselmann KF, Hendrickson CL, Marshall AG, Observation of Charge Localization Effect on Electron Capture Dissociation Fragmentation Pattern. Proceedings of the 53rd ASMS Conference on Mass Spectrometry and Allied Topics, San-Antonio, Texas, 5–9 June (2005) [Google Scholar]
- 30.Tsybin YO, Haselmann KF, Emmett MR, Hendrickson CL, Marshall AG: Charge location directs electron capture dissociation of peptide dications. J. Am. Soc. Mass Spectrom. 17, 1704–11 (2006) [DOI] [PubMed] [Google Scholar]
- 31.Kjeldsen F, Haselmann KF, Budnik BA, Jensen F, Zubarev RA: Dissociative capture of hot (3–13 eV) electrons by polypeptide polycations: an efficient process accompanied by secondary fragmentation. Chem. Phys. Lett. 356, 201–206 (2002) [Google Scholar]
- 32.Budnik BA, Nielsen ML, Olsen JV, Haselmann KF, Hörth P, Haehnel W, Zubarev RA: Can relative cleavage frequencies in peptides provide additional sequence information? Int. J. Mass Spectrom. 219,283–294 (2002) [Google Scholar]
- 33.Creese AJ, Cooper HJ: The effect of phosphorylation on the electron capture dissociation of peptide ions. J. Am. Soc. Mass Spectrom. 19,1263–1274 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


