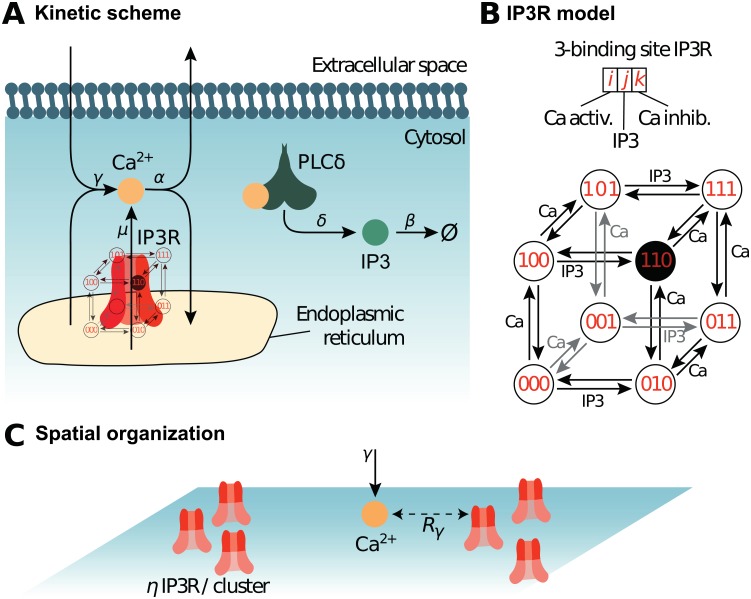
Fig 1. Reaction scheme and IP3R model.
The biochemical processes included in the model are illustrated in (A). Cytosolic calcium can exit the cytosol to the extracellular space or the endoplasmic reticulum (ER) at a (total) rate α, lumping together the effects of ER and plasma membrane pumps. Likewise, Ca2+ can enter the cytosol from the extracellular space or from the ER via IP3R-independent flow, with (total) rate γ, emulating calcium channels from the plasma membrane. When an IP3R channel opens, calcium enters the cytosol through the channel at rate μ. Phospholipase Cδ (PLCδ), once activated by calcium binding, produces IP3 at rate δ. Like Ca2+, IP3 can bind the IP3R channel and is removed with rate β. (B) Our model of the kinetics of the IP3R channel is an 8-state Markov model adapted from [46, 56]. Each IP3R channel monomer is associated with 3 binding sites, two calcium binding sites and one IP3 binding site. Occupancy states are designated by a triplet {i, j, k} where i stands for the occupation of the first Ca binding site (i = 1 if bound, 0 else), j for that of the IP3 binding site and k for the second Ca site. The first calcium binding site has higher affinity than the second. The open state is state {110}, where the first Ca and the IP3 sites are bound but not the second Ca site. (C) Spatial parameters for the particle-based model. The molecules are positioned within uniformly distributed clusters, with η IP3R in each cluster. Hence η = 1 corresponds to uniformly distributed IP3R (no clustering), while the degree of clustering increases with η (for constant total IP3R number). To account for potential co-localization between IP3R-dependent and IP3R-independent calcium sources, the influx of IP3R-independent calcium (at rate γ) occurs within distance Rγ of an IP3R. Thus, low values of Rγ emulate co-localization between IP3R-dependent and IP3R-independent Ca2+ influx sources.