Fig. 2. TtClpX activates TtClpP peptidase activity.
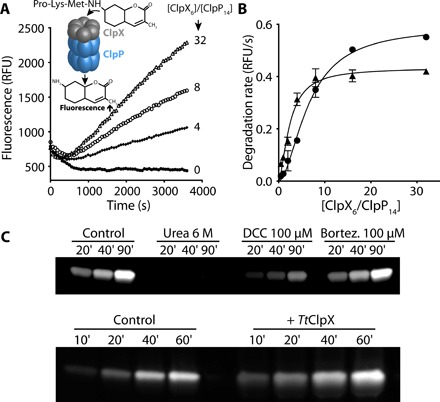
(A) TtClpP (0.1 μM complex) peptidase activity was measured in the presence of TtClpX at the indicated ClpX6/ClpP14 ratios. (B) The activation of TtClpP peptidase activity in the absence (circles) or presence (triangles) of bortezomib (10 μM) is plotted as a function of the molar ClpX6/ClpP14 ratio. Half-maximal peptidase activity was obtained at 6.5 ± 0.5 and 2.4 ± 0.3 ClpX6/ClpP14 ratio for the apo and bortezomib-loaded TtClpP, respectively. (C) Activation of the TtClpP active site by bortezomib (100 μM) and TtClpX (1 μM), monitored by labeling with TAMRA-FP. TAMRA-FP (5 μM) was incubated with TtClpP (1 mg/ml), and aliquots of the reaction mixture were removed at the indicated time points. Labeling of the active-site serines is reflected by the increase in fluorescence observed.
