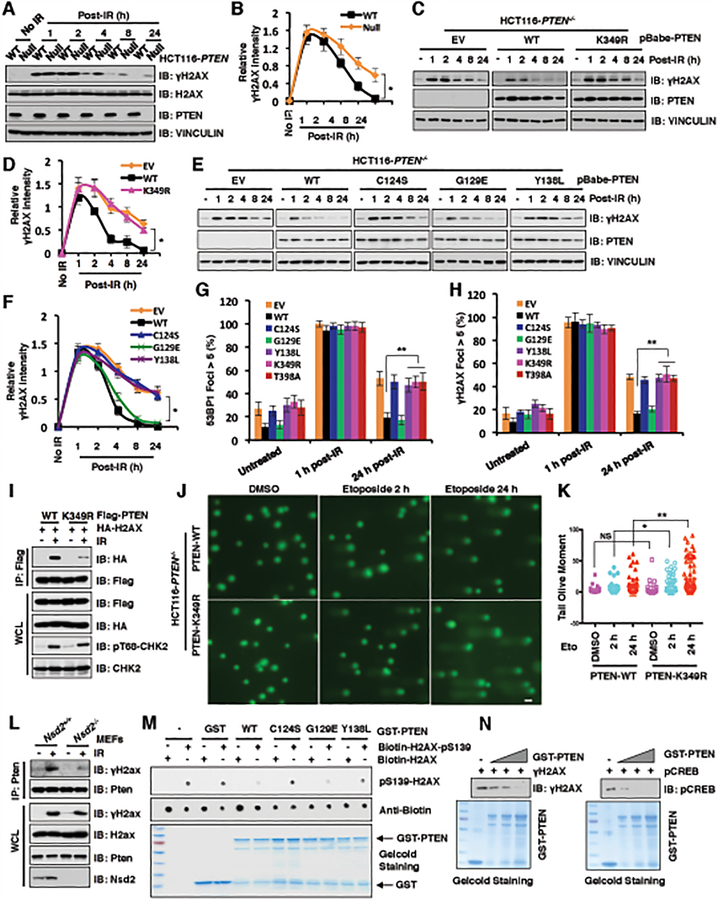
Figure 4. PTEN K349 di-methylation and protein phosphatase activity are required for efficient DSBs repair.
(A and B) PTEN deficiency elevated γH2AX after 4 h post-irradiation (IR) treatment. IB analysis of WCL derived from PTEN+/+ and PTEN−/− HCT116 cells after treatment with IR (5 Gy) as indicated time points (A). Quantification of protein intensity was performed using the ImageJ software (B). γH2AX immunoblot bands were normalized to VINCULIN, and then normalized to the control (no IR treatment). Data are represented as mean ± s.d., n = 3. *p < 0.05 (Student’s t-test).
(C and D) PTEN wild type (WT), but not K349R mutant, rescued PTEN deficiency mediated high levels of γH2AX after 4 h post-IR treatment. IB analysis of WCL derived from PTEN−/− HCT116 cells introducing PTEN WT, K349R as well as EV, which were treated with IR (5 Gy) at indicated time points before harvesting (C). Quantification of protein intensity was performed using the ImageJ software (D). γH2AX immunoblot bands were normalized to VINCULIN, and then normalized to the control (no IR treatment). Data are represented as mean ± s.d., n = 3. *p < 0.05 (Student’s t-test).
(E and F) PTEN protein phosphatase activity, but not lipid phosphatase activity, was required for regulating γH2AX levels. IB analysis of WCL derived from PTEN−/− HCT116 cells re-introducing PTEN WT, C124S, G129E, Y138L as well as EV, which were treated with IR (5 Gy) at indicated time points before harvesting (E). Quantification of protein intensity was performed using the ImageJ software (F). γH2AX immunoblot bands were normalized to VINCULIN, then normalized to the control (no IR treatment). Data are represented as mean ± s.d., n = 3. *p < 0.05 (Student’s t-test).
(G and H) PTEN−/− HCT116 cells reconstituted with the indicated PTEN constructs were treated with IR (5 Gy) and immunostained at the indicated times with anti-53BP1 or anti-γH2AX. Quantification of 53BP1 (G) or γH2AX (H) foci positive cells (foci > 5 per cell) was performed by counting a total of 100 cells per sample, respectively. Data are represented as mean ± s.d., n = 3, and **p < 0.01 (Student’s t-test).
(I) PTEN-K349R mutant decreased its interaction with H2AX. IB analysis of anti-Flag IPs and WCL derived from U2OS cells transfected with the indicated constructs. 36 h after transfection, cells were harvested for IP assays after treatment with IR (5 Gy) for 60 min.
(J and K) PTEN-K349R mutation attenuated DNA damage repair. PTEN-WT or PTEN-K349R mutant expressed HCT116 cells were treated with DMSO or etoposide for 2h and 24h, respectively. (J) Images were captured following Comet assay; and (K) Tail olive moment values were calculated using Casplab software. Error bars represent standard error of the mean. **p<0.01, *p<0.05. n=80. Scale bar: 50 μM.
(L) Nsd2 deficiency disrupted Pten interaction with γH2ax. IB analysis of anti-Pten IPs and WCL derived from Nsd2+/+ and Nsd2−/− MEFs treatment with/without IR (5 Gy) for 60 min before harvesting.
(M and N) In vitro dephosphorylation assays were performed with bacterially purified recombinant GST-tagged PTEN WT and the indicated PTEN mutants including C124S, G129E, and Y138L incubating with indicated H2AX synthetic peptides (M) or purified γH2AX (N) from HCT116 cells after etoposide treatment, then analyzed by immunoblot analyses.